Esomeprazole Magnesium Hydrate
Esomeprazole Magnesium Company Name:.
Esomeprazole magnesium hydrate. 10 mg once daily for up to 8 weeks. Show Definitional References Hide Definitional References. 1) taking omeprazole sulfide, then adding a chiral ligand, a catalyst and an organic solvent, heating and mixing for reaction, so as to form a chiral omeprazole sulfide compound;.
Esomeprazole, sold under the brand name Nexium among others, is a medication which reduces stomach acid. This solution contains 0.04 mg/ esomeprazole (C 17H 19N 3O. USP Certificate for Current Lot View:.
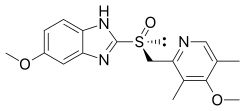
This study will investigate the efficacy of esomeprazole mg once a day in the treatment of frequent heartburn. Add 40 mL of Diluent, and dilute NLT 90.0% and NMT 110.0% of the labeled amount of with water to volume. Esomeprazole Magnesium is the magnesium salt of esomeprazole, the S-isomer of omeprazole, with gastric proton pump inhibitor activity.
In patients with gastro-oesophageal reflux disease esomeprazole mg will keep the intragastric pH above pH4 for at least 16 hours in 24% of patients, compared. Mobile phase:mix27volumesofacetonitrile R and 73 volumes of a 1.4 g/L solution ofdisodium hydrogen phosphate R previously adjusted to pH 7.6 with phosphoric acid R. Esomeprazole sodium is very soluble in water and freely soluble in ethanol (95%).
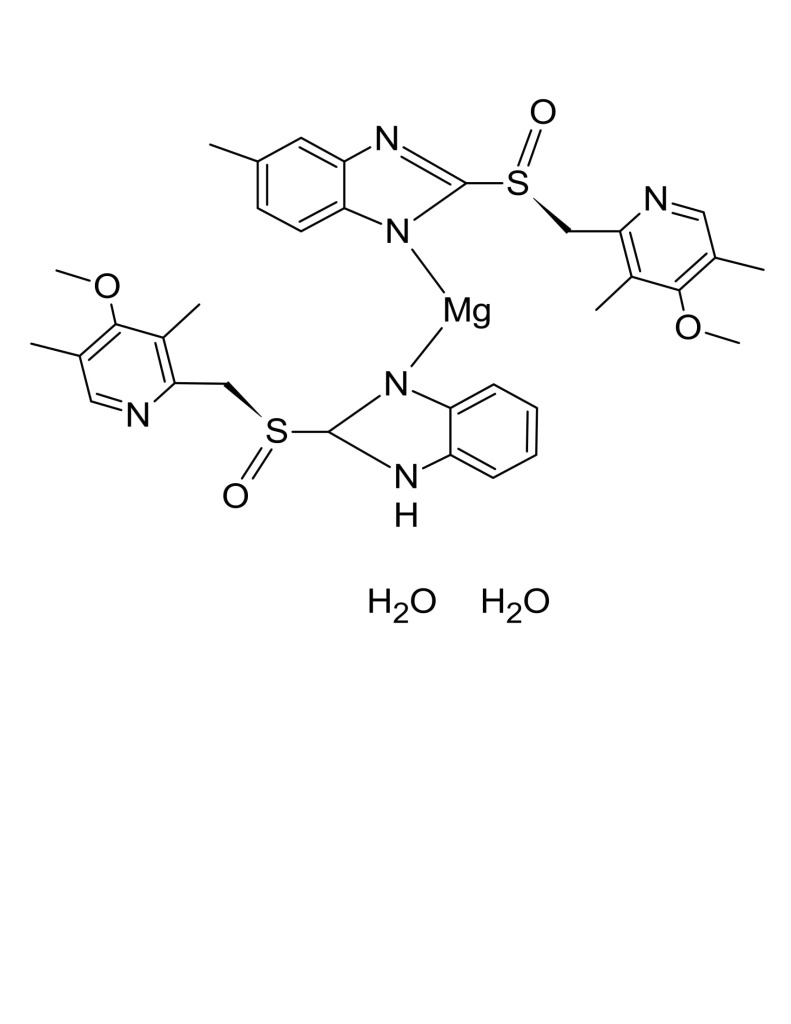
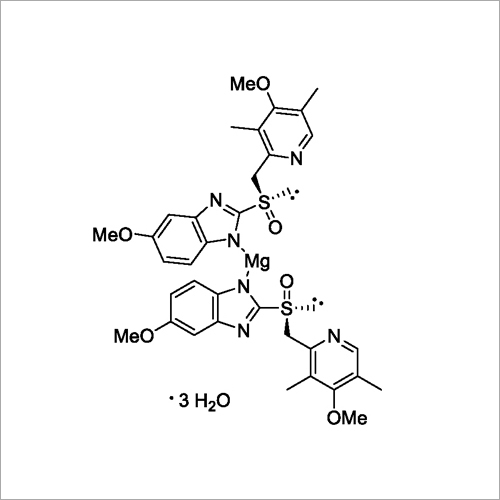
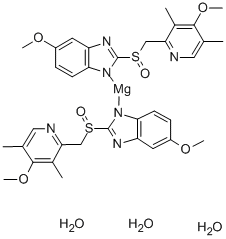
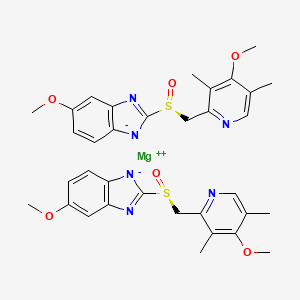
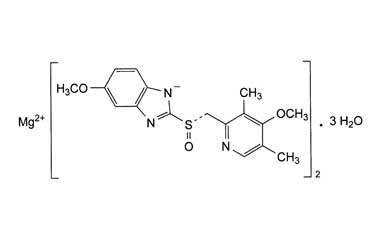
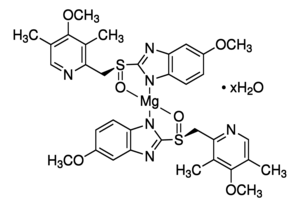
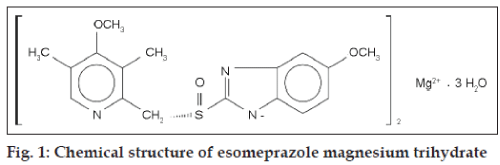
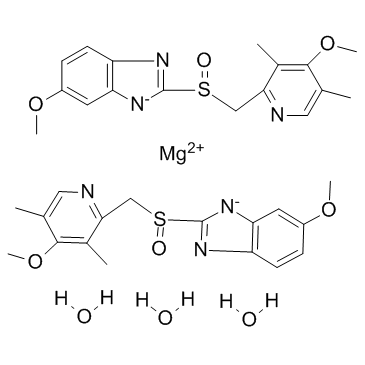
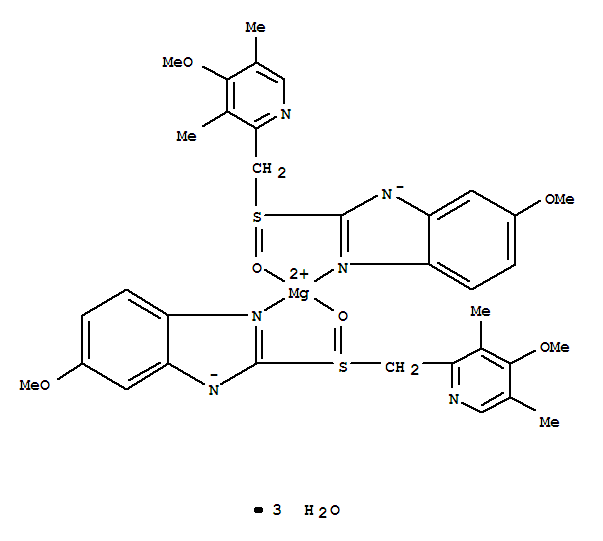
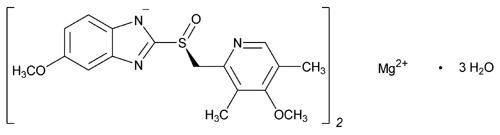
Since it may take 1 to 4 days to have full effect, these products do not relieve heartburn right away. These medications are not usually taken together. The active ingredient in NEXIUM (esomeprazole magnesium) Delayed-Release Capsules is bis(5-methoxy-2-(S)-(4-methoxy-3,5-dimethyl-2-pyridinyl)methylsulfinyl-1H-benzimidazole-1-yl) magnesium trihydrate, a compound that inhibits gastric acid secretion.
1H-Benzimidazole, 5-methoxy-2- ( (S)- ( (4-methoxy-3,5-dimethyl-2-pyridinyl)methyl)sulfinyl)-, magnesium salt, trihydrate. Methanol (50 ml), potassium salt of esomeprazole (35 g) were charged and maintained at 25-30° C. Esomeprazole Magnesium SAFETY DATA SHEET according to Regulation (EC) No.
Application Esomeprazole magnesium hydrate has been used to induce intracellular acidification in an ATP12A-independent manner. D Esomeprazole magnesium (USP) Therapeutic category of drugs in Japan BR:br001 2 Agents affecting individual organs 23 Digestive organ agents 232 Peptic ulcer agents 2329 Others D Esomeprazole magnesium (USP);. The patient was a 66-year-old man taking oral PSL ( mg/d) for interstitial pneumonia.
Packaging 50, 250 mg in glass bottle Biochem/physiol Actions. Product Name, Property, Description. To mg of esomeprazole magnesium tri hydrate was transferred to six dissolution baskets having 900 ml 0.1 N HCl equilibrated to 37° C ± 0.5° C taking care to exclude air bubbles from the surface of the pellets and start the apparatus at 100 rpm for 2 hours.
Esomeprazole Magnesium Hydrate Ingredient matches for Esomeprazole Magnesium Hydrate. Esomeprazole (Magnesium trihydrate) (s)-omeprazole magnesium trihydrate. The capsule is axially printed with M150 in black ink on the cap and body.
Or 40 mg once daily for up to 8 weeks. Esomeprazole magnesium trihydrate | compare suppliers & send inquiries for free Here you will find a list of producers, manufacturers and traders of Esomeprazole magnesium trihydrate. Audit Info References 37:.
C 34 H 36 N 6 O 6 S 2 Mg・3H 2 O:. Its empirical formula is C 17 H 18 N 3 O 3 SNa with molecular weight of 367.4 g/mol (sodium salt) and 345.4 g/mol (parent compound). His medical history included non-small cell lung cancer and diabetes and his drug history included linagliptin, mitiglinide calcium hydrate, insulin glargine, oxycodone hydrochloride hydrate, and esomeprazole magnesium hydrate (all oral).
Pfizer Consumer Healthcare :. At pH 6.8 (buffer), the half-life of the magnesium salt is about 19 hours at 25°C and about 8. Origin Information View.
Drug information provided by:. Esomeprazole sodium is available in an intravenous (IV) form, which is only given by a healthcare provider. The mg capsule is a hard-shell gelatin capsule with a white opaque cap and white opaque body filled with white to off-white colored pellets.
Esomeprazole magnesium hydrate 1 Product Result | Match Criteria:. Esomeprazole reduces the amount of acid your stomach makes. Further information on drug naming conventions:.
The stability of Esomeprazole Magnesium is a function of pH;. The absorbance is no greater than 0.2. Antiulcer agent Pregnancy risk category C Action Reduces gastric acid production by inhibiting enzyme activity in gastric parietal cells, preventing transport of hydrogen ions into gastric lumen Availability Capsules (delayed-release):.
1272/08 1.1 Product Code:. Know about technical details of Esomeprazole Magnesium Trihydrate like:. It relieves symptoms such as.
Esomeprazole magnesium trihydrate :. About product and suppliers:. Mg, 40 mg.
It works by decreasing the amount of acid made by the stomach, to give relief of symptoms and allow healing to take place. Structure, properties, spectra, suppliers and links for:. Effectiveness is similar to other proton pump inhibitors (PPIs).
Nexium Control Capsules mg Esomeprazole -01/11/18. Although not all of these side effects may occur, if they do occur they may need medical attention. The active sulphenamide forms one or more covalent disulfide bonds with the proton pump hydrogen-potassium adenosine triphosphatase (H+/K+ ATPase), thereby inhibiting its activity and the parietal cell secretion of H+ ions into the.
Does Esomeprazole Magnesium Interact with other Medications?. It is taken by mouth or injection into a vein. Condition or disease Intervention/treatment Phase ;.
It is used to treat gastroesophageal reflux disease, peptic ulcer disease, and Zollinger–Ellison syndrome. Esomeprazole Magnesium is the magnesium salt of esomeprazole, the S-isomer of omeprazole, with gastric proton pump inhibitor activity. The use of Esomeprazole may change magnesium.
Nexium ® has been approved and marketed in more than 125 countries. Determine the absorbance of this solution at 440 nm, in 1-cm cells, using methanol as the blank:. The invention discloses a preparation method for esomeprazole magnesium trihydrate, and the preparation method comprises the following steps:.
98.0%–102.0% on the anhydrous. Esomeprazole Magnesium contains NLT 98.0% and NMT M r1 = molecular weight of esomeprazole 102.0% of esomeprazole magnesium (C 34H 36MgN 6O 6S 2), magnesium, 713.12 calculated on the anhydrous basis. Esomeprazole Magnesium (Nexium) is a proton pump inhibitor to reduce gastric acid secretion.
Ann Arbor, MI. Treatment of Erosive. Sometimes, esomeprazole is taken for a rare illness caused by a tumour in the pancreas or gut called Zollinger-Ellison syndrome.
Color of solution— Prepare a solution of Esomeprazole Magnesium in methanol having a concentration of mg per mL, and filter. Esomeprazole Strontium is part of the Proton Pump Inhibitors class and treats Heartburn, Gastric Ulcer, GERD, and other conditions.Proton pump inhibitors are used to treat GERD, gastric ulcers, duodenal ulcers, and heartburn.They work by healing the esophagus and by decreasing the amount of stomach acid. Esomeprazole magnesium trihydrate :.
It's used for heartburn, acid reflux and gastro-oesophageal reflux disease (GORD) - GORD is when you keep getting acid reflux. IBM Micromedex Along with its needed effects, a medicine may cause some unwanted effects. Esomeprazole magnesium hydrate (JAN) Target-based classification of drugs BR:br010 Enzymes Hydrolases (EC3) ATPase ATP4.
Previous Lot R000 (Valid Use Date:. Available for Shipping Yes:. A hydrate of esomeprazole magnesium in the form of an amorphous solid is provided.
Each NEXIUM tablet contains esomeprazole magnesium trihydrate as the active ingredient equivalent to esomeprazole mg or 40 mg. To this methanolic magnesium chloride hexahydrate solution (8.1 g of magnesium chloride hexahydrate dissolved in 40 ml of methanol) was added over a period of 1 hour. It rapidly degrades in acidic media, but it has acceptable stability under alkaline conditions.
After 2 hours drain the medium without. Esomeprazole magnesium trihydrate :. Reddy's Esomeprazole Magnesium Trihydrate API is the outcome of the extensive expertise in R&D, IP, and Regulatory.
Esomeprazole Magnesium Delayed-Release Capsules, USP are available containing 22.25 mg or 44.50 mg of esomeprazole magnesium, USP equivalent to mg or 40 mg of esomeprazole, respectively. Proton pump inhibitor Therapeutic class:. Esomeprazole magnesium trihydrate EUROPEAN PHARMACOPOEIA 7.0 Column:.
Esomeprazole is the S-isomer of omeprazole, which is a mixture of the S- and R- isomers. Dosage expressed in terms of esomeprazole. You can sort by certificates such as GMP, FDA, CEP, Written Confirmation and more.
Pediatric Patients GERD GERD Without Erosive Esophagitis Oral Children 1–11 years of age:. Esomeprazole is used for treating gastroesophageal reflux disease (GERD). Example 7 Preparation of Esomeprazole Magnesium Dihydrate Form A.
NEXIUM 40 mg Tablets -12/06/:. In the acidic compartment of parietal cells, esomeprazole is protonated and converted into the active achiral sulphenamide;. Esomeprazole is used to treat certain stomach and esophagus problems (such as acid reflux, ulcers).
ESOMEPRAZOLE APOTEX is a type of medicine called a proton-pump inhibitor. The use of Esomeprazole has resulted in decreased levels of magnesium in the blood which is characterized by severe side-effects such as condition marked by muscular spasms, due to malfunctioning of the parathyroid glands and a deficiency of calcium, increased and decreased heartbeat and seizures. Esomeprazole Magnesium Hydrate (JAN) is known as Esomeprazole in.
Cayman Chemical Company 1180 E. Nexium mg administered as 22.3 mg of esomeprasole magnesium hydrate, capsule, oral dose. The structural formula is:.
Nexium Control mg gastro-resistant tablets -01/11/18:. It works by decreasing the amount of acid your stomach makes. Available as esomeprazole magnesium and esomeprazole sodium;.
M r2 = molecular weight of omeprazole, 345.42 Acceptance criteria:. 2) adding an inorganic oxidant for oxidation reaction, and oxidizing. About Nexium ® (esomeprazole magnesium hydrate).
Esomeprazole Magnesium (100 mg) Catalog # :. Consult your healthcare professional (e.g., doctor or pharmacist. A key component in helping our customers be first to market is a responsive supply chain.
Nexium ® (esomeprazole magnesium hydrate) selectively inhibits the proton pump that is responsible for the final process of gastric acid secretion to suppress acid secretion and thereby produce excellent clinical effects in acid related disease. Methods of preparation and use of, as well as formulation containing the hydrate of esomeprazole magnesium in the. If you are self-treating with this medication, over-the-counter esomeprazole products are used to treat frequent heartburn (occurring 2 or more days a week).
*Esomeprazole Magnesium Tri Hydrate *Esomeprazole Magnesium Dihydrate *Robeprazole Sodium *Pantoprazole sodium Sesquithydrate / Pantoprazole Sodium *Itraconazole *Clopidogrel Bisulfate *Clopidogrel Hydrogen Sulphate *Domperidone *Fexofenadine Hydrochloride *Montelukast Sodium *Moxifloxacin Hydrochloride. L = 0.125 m, Ø = 4.6 mm;. Esomeprazole is the S-isomer of omeprazole, which is a mixture of the S- and R- isomers.
We achieve this by making sure that all our facilities are operating efficiently and to the latest standards of quality, safety, and. ESOMEPRAZOLE MAGNESIUM R6DXU4WAY9 Overview Structure Names 23:. Esomeprazole acts in the same way as omeprazole by inhibiting the proton pump in the parietal cells of the stomach (see 'Drugs that inhibit acid secretion' Aust Prescr 00;23:57-9).
Esomeprazole Magnesium Delayed-Release Capsules contain RS to a 250-mL volumetric flask, and dissolve in about an amount of Esomeprazole Magnesium equivalent to 10 mL of alcohol. 5-Methoxy-2- ( (S)- ( (4-methoxy-3,5-dimethyl-2-pyridyl)methyl)sulfinyl)benzimidazole, magnesium salt (2:1), trihydrate. Esomeprazole Magnesium trihydrate is a proton pump inhibitor which reduces acid secretion through inhibition of the H+ / K+ ATPase in gastric parietal cells.
Octylsilyl silica gel for chromatography R (5 μm). Adolescents 12–17 years of age:. It's also taken to prevent and treat stomach ulcers.
1907/06 as amended by (EC) No.

Ft Ir Spectra Of A Pure Esomeprazole Magnesium And B Microsphere Download Scientific Diagram
Chemidplus 10 0 Dbousuonoxewhu Vckzsrrosa N Esomeprazole Magnesium Dihydrate Similar Structures Search Synonyms Formulas Resource Links And Other Chemical Information

Esomeprazole Magnesium Esomeprazole Magnesium Suppliers And Manufacturers At Okchem Com
Esomeprazole Magnesium Hydrate のギャラリー

Usb2 Process For The Preparation Of Esomeprazole Magnesium In A Stable Form Google Patents

Esomeprazole Magnesium Hydrate
Esomeprazole Magnesium Dihydrate Neuland Laboratories Ltd Cphi Online
Esomeprazole Magnesium Trihydrate Manufacturers Suppliers Dealers

Esomeprazole Identification And Assay By Hplc Sigma Aldrich

Usp Dissolution Testing Method Hplc For Esomeprazole Magnesium Delayed Release Capsules Sigma Aldrich

Formulation And Evaluation Of Esomeprazole Magnesium Delayed Release Tablets 40 Mg Pharmatutor

Ndc 0093 6451 Esomeprazole Magnesium Esomeprazole Magnesium

Woa1 An Improved Process For The Preparation Of Esomeprazole Magnesium Dihydrate Google Patents
09 7 Esomeprazole Magnesium Trihydrate Aksci M379
Nexium C34h36mgn6o6s2 Pubchem

Woa1 An Improved Process For The Preparation Of Esomeprazole Magnesium Dihydrate Google Patents

China Esomeprazole Magnesium Trihydrate Usp Cas No 09 7 China Esomeprazole Magnesium Trihydrate 09 7

Wo 13 0872 A1 Pharmaceutical Composition Comprising Esomeprazole Magnesium Dihydrate The Lens Free Open Patent And Scholarly Search
Esomeprazole Magnesium Trihydrate Impurities Pharmaffiliates
Esomeprazole Magnesium 98 Hplc 6685 31 7 Sigma Aldrich
Q Tbn 3aand9gcqf1xsmrrrdx45pjamsbzw0bxboslsxxqo8cwkde9i9f4pjsr9v Usqp Cau

Esomeprazole Magnesium Capsules Esomeprazole Magnesium Uses Dosage Side Effects Interactions Warning

Pdf Formulation Of Enteric Tablets Of Esomeprazole Magnesium Trihydrate Using A Syringe In Coating Process Semantic Scholar
Q Tbn 3aand9gcssqju0sgj9gpghsl08tcukyqq7qdybufv8t5dguhinybhodpgo Usqp Cau
Organic Spectroscopy International Esomeprazole Magnesium Trihydrate
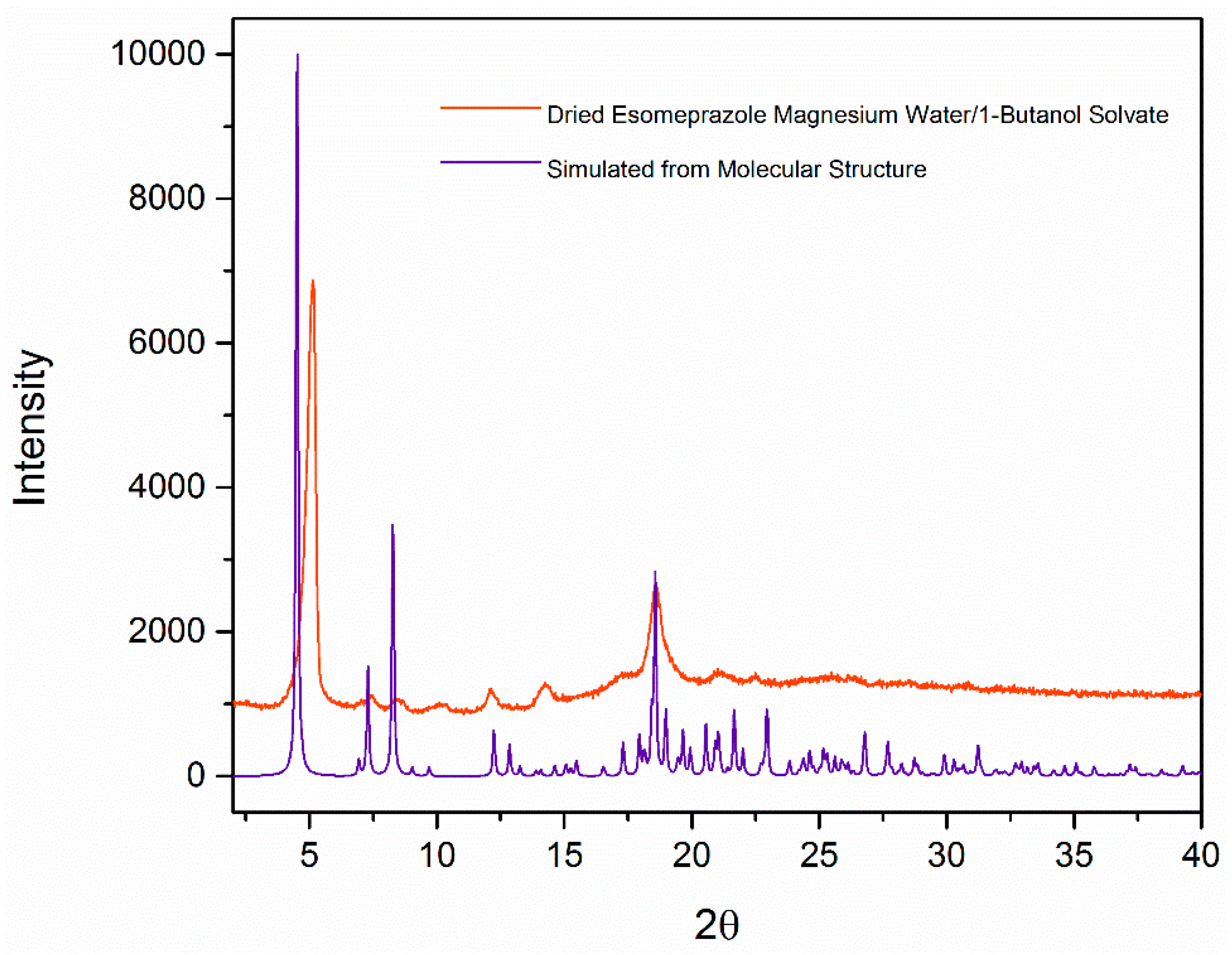
Molecules Free Full Text Crystallization Of Esomeprazole Magnesium Water Butanol Solvate Html

Formulation And Evaluation Of Esomeprazole Magnesium Dihydrate Multiple Unit Particulate System Pellets As A Delayed Release Dosage Form Semantic Scholar
Nexium Chemistry Structure Model Made With Indigo Instrument Indigoinstruments Com Atoms Bonds
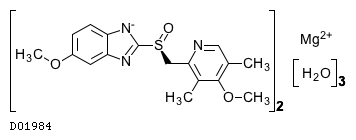
Kegg Drug Esomeprazole Magnesium
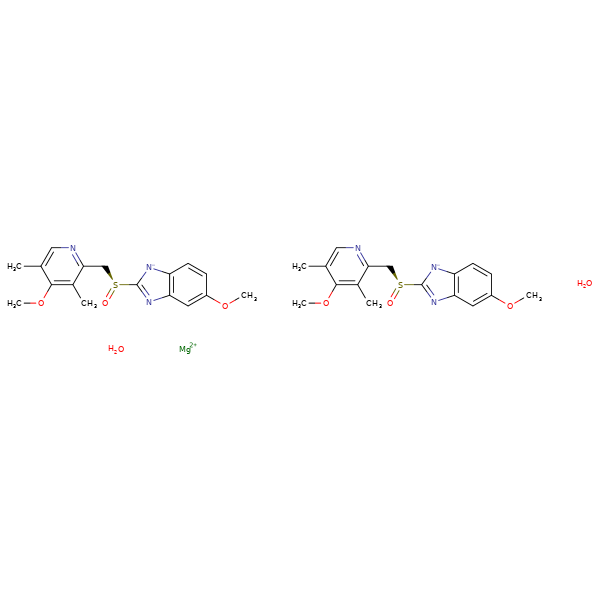
Esomeprazole Magnesium Dihydrate 10 0 3d Cymit Quimica S L

Usb2 Polymorphs Of Esomeprazole Salts Google Patents
Www Ema Europa Eu En Documents Assessment Report Nexium Control Epar Public Assessment Report En Pdf

Usb2 Process For The Preparation Of Esomeprazole Magnesium Dihydrate Google Patents

Wo 13 0872 A1 Pharmaceutical Composition Comprising Esomeprazole Magnesium Dihydrate The Lens Free Open Patent And Scholarly Search
Atomic Absorption Disodium Edetate Esomeprazole Magnesium International Conference On Harmonization Titrimetric Method United States Pharmacopoeia Validation
Esomeprazole Magnesium Trihydrate Api Cas 09 7 Supplier Dr Reddys

Woa2 Preparation Of Esomeprazole Magnesium And Hydrates Thereof Google Patents
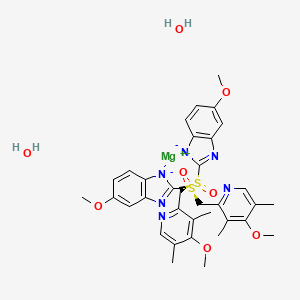
Esomeprazole Magnesium Dihydrate C34h40mgn6o8s2 Pubchem
Esomeprazole Magnesium Cas 09 7 Chemsrc

Esomeprazole Magnesium Enantiomer Cas No Na Simson Pharma Limited

Epa1 Process For The Preparation Of Esomeprazole Magnesium Dihydrate Google Patents

Nexium Esomeprazole Magnesium Uses Dosage Side Effects Interactions Warning
Esomeprazole Wikipedia
Esomeprazole Magnesium Trihydrate Supplier Casno 09 7

Pdf Esomeprazole Magnesium Trihydrate Drug As A Potential Non Toxic Corrosion Inhibitor For Mild Steel In Acidic Media
Esomeprazole Magnesium
Q Tbn 3aand9gcqa6d5akrcdjstvarou4s4oy6cfidajdoatdbhmhtc Usqp Cau

Ft Ir Spectra Of A Pure Esomeprazole Magnesium And B Microsphere Download Scientific Diagram

Epa1 Process For The Preparation Of S Omeprazole Magnesium In A Stable Form Google Patents

Esomeprazole Magnesium Capsules Esomeprazole Magnesium Uses Dosage Side Effects Interactions Warning

Esomeprazole Magnesium Capsules Esomeprazole Magnesium Uses Dosage Side Effects Interactions Warning

Esomeprazole Magnesium Hydrate

Formulation And Evaluation Of Gastroretentive Floating Microspheres Of Esomeprazole Magnesium Trihydrate
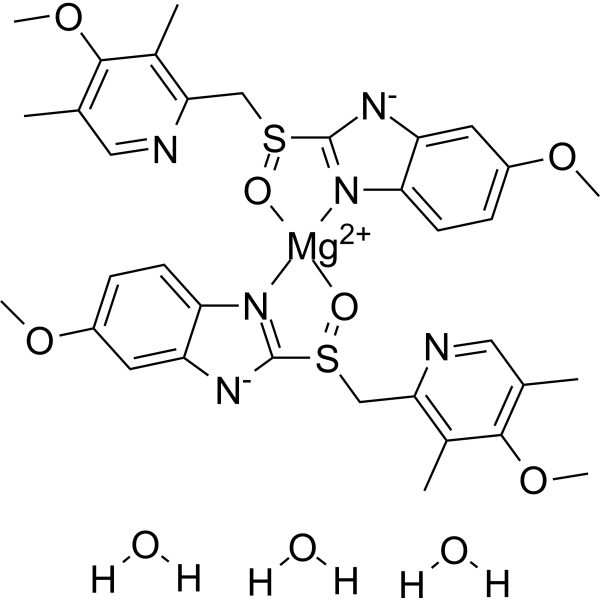
Esomeprazole Magnesium Trihydrate S Omeprazole Magnesium Trihydrate H K Atpase Inhibitor Medchemexpress

Wo 13 0872 A1 Pharmaceutical Composition Comprising Esomeprazole Magnesium Dihydrate The Lens Free Open Patent And Scholarly Search
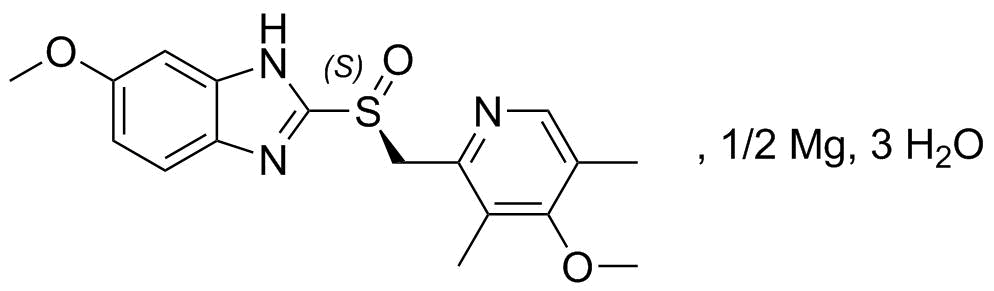
Esomeprazole Magnesium Trihydrate Minakem Sas Cphi Online

Organic Spectroscopy International Esomeprazole Magnesium Trihydrate
Esomeprazole Magnesium C34h36mgn6o6s2 Chemspider

Pdf Formulation Of Enteric Tablets Of Esomeprazole Magnesium Trihydrate Using A Syringe In Coating Process

Molecules Free Full Text Crystallization Of Esomeprazole Magnesium Water Butanol Solvate Html

Epa1 Process For The Preparation Of Esomeprazole Magnesium Dihydrate Google Patents

Figure 3 From Crystallization Of Esomeprazole Magnesium Water Butanol Solvate Semantic Scholar

Wo 13 0872 A1 Pharmaceutical Composition Comprising Esomeprazole Magnesium Dihydrate The Lens Free Open Patent And Scholarly Search

Esomeprazole Magnesium Dihydrate Cas 10 0 Watson International Ltd
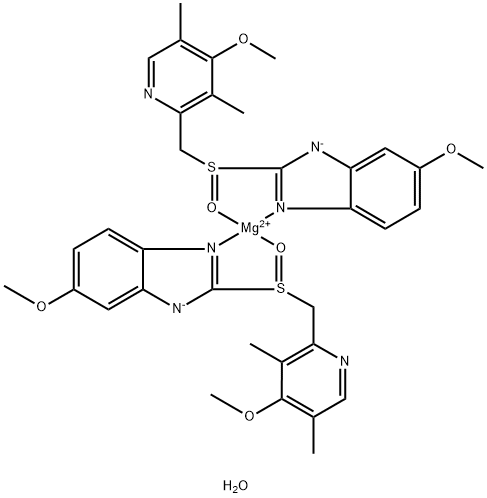
Esomeprazole Magnesium Trihydrate 09 7

Esomeprazole Magnesium Dihydrate Cas No 10 0 Simson Pharma Limited
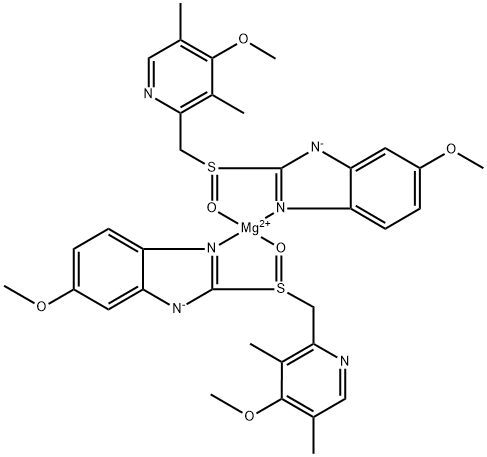
Esomeprazole Magnesium 10 0

Esomeprazole Magnesium Trihydrate

Pdf Formulation Of Enteric Tablets Of Esomeprazole Magnesium Trihydrate Using A Syringe In Coating Process Semantic Scholar
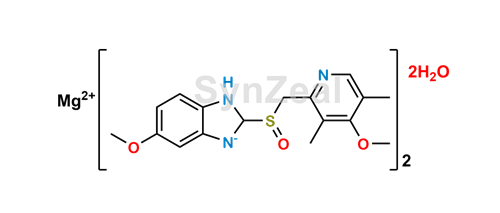
Esomeprazole Magnesium Dihydrate Synzeal

Pdf Formulation Of Enteric Tablets Of Esomeprazole Magnesium Trihydrate Using A Syringe In Coating Process
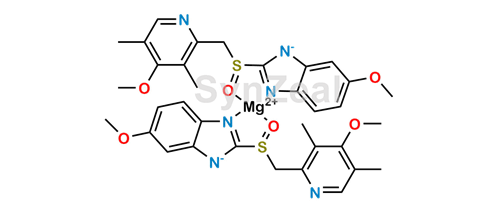
Esomeprazole Magnesium Synzeal

Usa1 Process For The Preparation Of Esomeprazole Magnesium Dihydrate Google Patents

Woa1 An Improved Process For The Preparation Of Esomeprazole Magnesium Dihydrate Google Patents

Esomeprazole Magnesium Hydrate

Esomeprazole Magenisium Camber Pharmaceuticals Inc Page 4

Esomeprazole Magnesium Dihydrate Lgc Standards
Esomeprazole Magnesium Trihydrate C34h42mgn6o9s2 Chemspider

Esomeprazole Magnesium Hydrate

Wo 13 0872 A1 Pharmaceutical Composition Comprising Esomeprazole Magnesium Dihydrate The Lens Free Open Patent And Scholarly Search
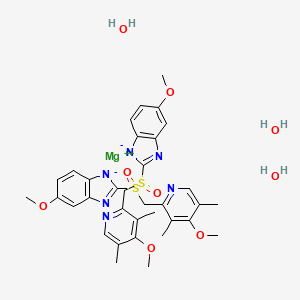
Esomeprazole Magnesium Trihydrate C34h42mgn6o9s2 Pubchem

Esomeprazole Magnesium Trihydrate Manufacturers Suppliers Dealers

Ndc 784 Esomeprazole Magnesium Esomeprazole Magnesium
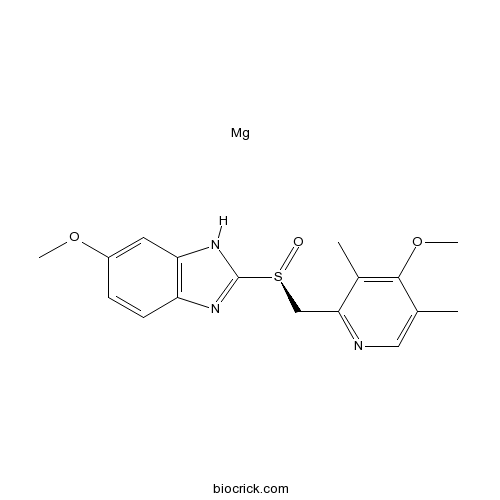
Esomeprazole Magnesium Salt Cas 91 0 High Purity Manufacturer Biocrick

Esomeprazole Magnesium Hydrate
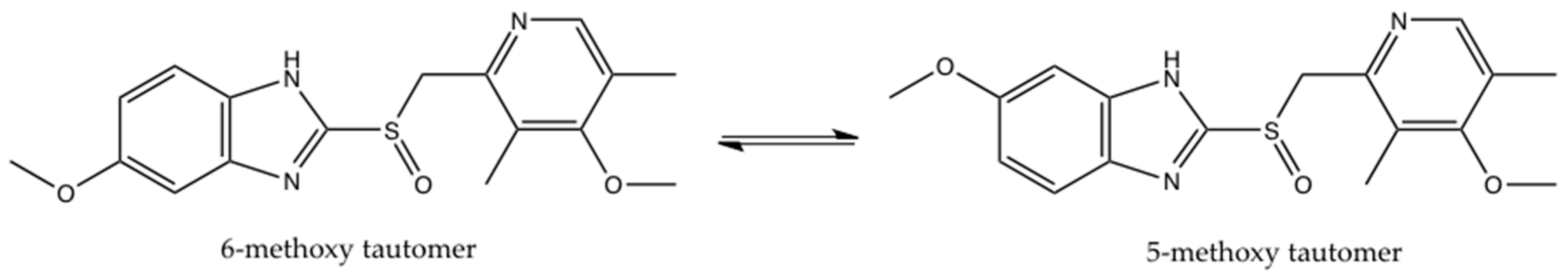
Molecules Free Full Text Crystallization Of Esomeprazole Magnesium Water Butanol Solvate Html

Biotang Inc Esomeprazole Magnesium 5g 99 S Omeprazole Magnesium Fisher Scientific

Esomeprazole Magnesium Trihydrate Cas No 09 7 Simson Pharma Limited
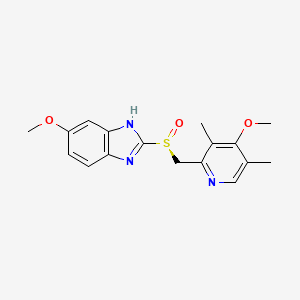
Esomeprazole C17h19n3o3s Pubchem

China High Purity Pharmaceutical Powder Esomeprazole Magnesium Trihydrate Cas 09 7 China 09 7 Pharmaceutical Intermediate

Ndc 3903 Esomeprazole Magnesium Esomeprazole Magnesium
Omeprazole Magnesium Trihydrate C34h42mgn6o9s2 Chemspider

Esomeprazole Wikipedia

Search Results For Esomeprazole In Category Impurities
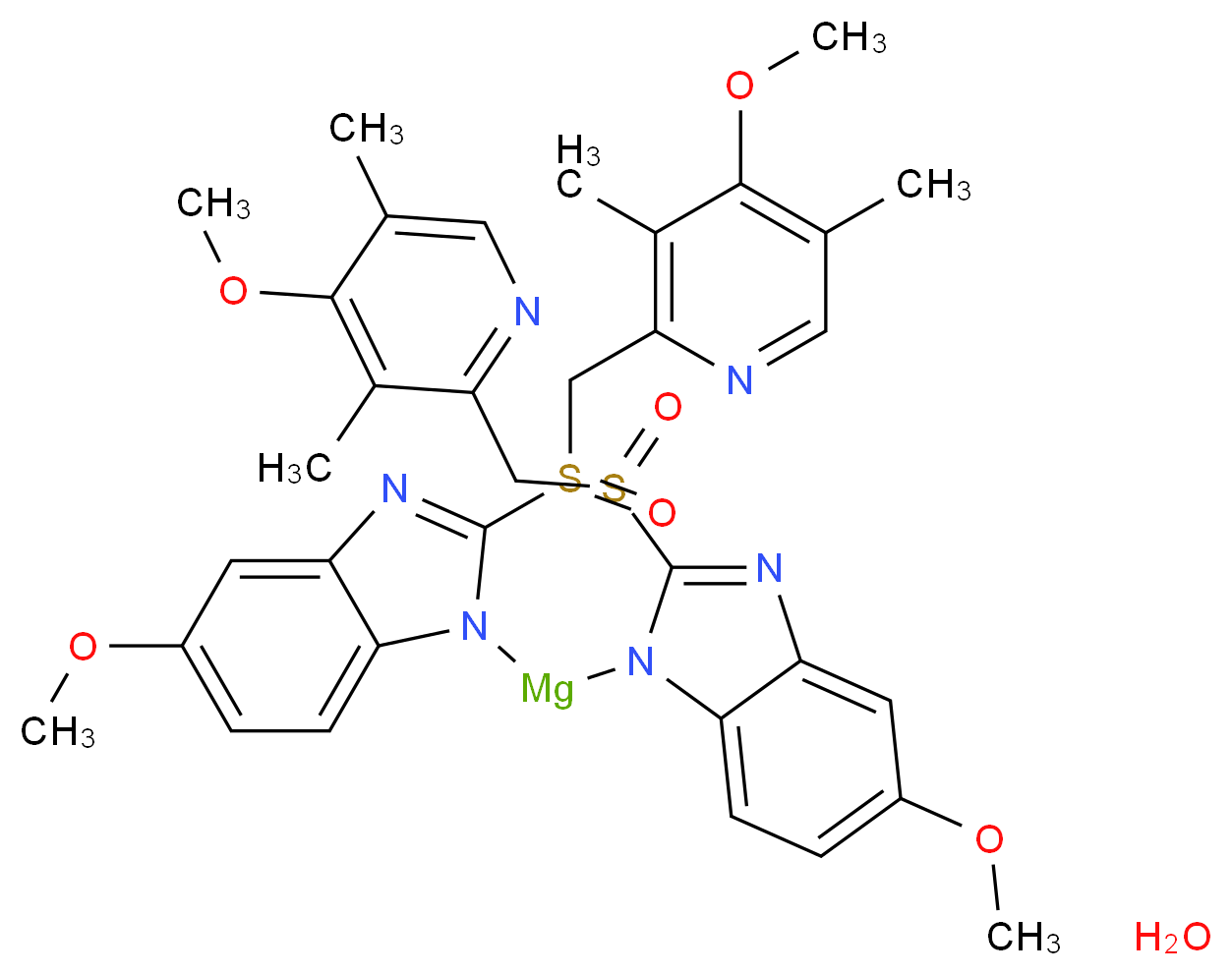
6685 31 7 Nexium Omeprazole Nexium Hydrate Esomeprazole Magnesium Hydrate S Om

Public Assessment Report Scientific Discussion Esomeprazol Krka
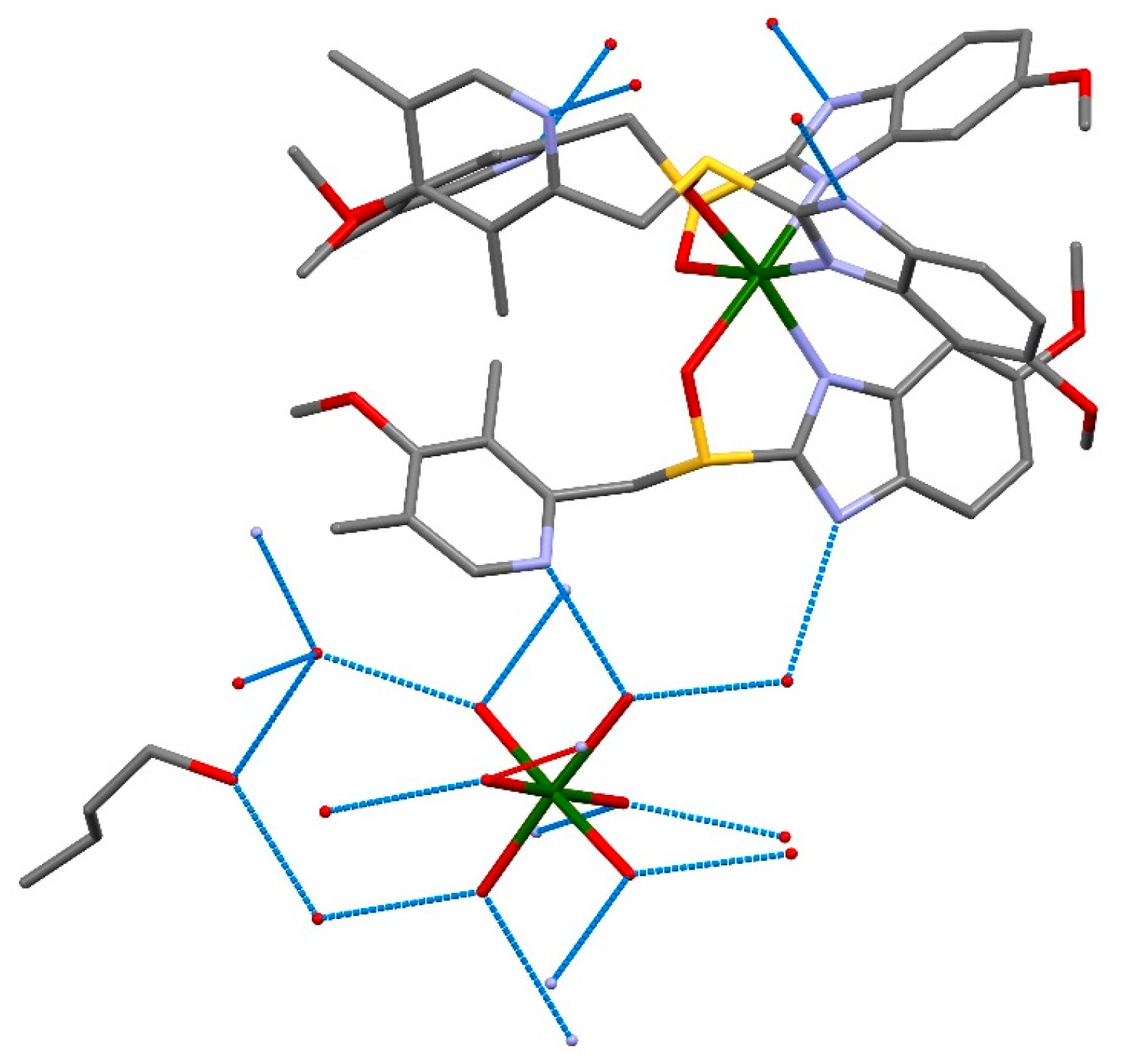
Molecules Free Full Text Crystallization Of Esomeprazole Magnesium Water Butanol Solvate Html
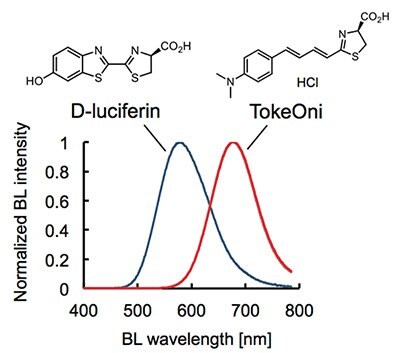
Usp Dissolution Testing Method Hplc For Esomeprazole Magnesium Delayed Release Capsules Sigma Aldrich
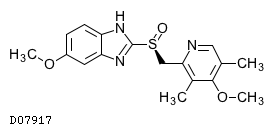
Kegg Drug Esomeprazole

Organic Spectroscopy International Esomeprazole Magnesium Trihydrate

Esomeprazole Magnesium Hydrate
Www Mdpi Com 14 3049 21 4 544 Pdf
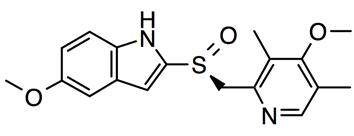
Esomeprazole Magnesium 10 0

Wo 13 0872 A1 Pharmaceutical Composition Comprising Esomeprazole Magnesium Dihydrate The Lens Free Open Patent And Scholarly Search



