Mek Inhibitor
June 27, 18—Today, the U.S.
Mek inhibitor. MEK inhibitors are approved to treat melanoma and other types of cancer, but in recent years researchers have found them to be effective in treating some brain tumors and. 92 The most common adverse effects of MEK inhibitors (e.g., trametinib) are rash, diarrhea, peripheral edema, fatigue, and dermatitis acneiform. The liquid was then aspirated from the wells and discarded.
BRAF & MEK Kinase Inhibitors The BRAF and MEK genes are known to play a role in cell growth, and mutations of these genes are common in several types of cancer. MEK inhibitors, when used as single agents, show no activity in BRAF -mutant melanoma that has become refractory to BRAF inhibitors and should not be used in this setting. This is where it becomes fascinating, so grab a coffee and switch off the phone because there is good news down the line.
The first use of MEK inhibitors as a potential treatment for NF tumors came from early-stage discoveries by Children's Tumor Foundation (CTF)-funded researchers, who showed that MEK inhibitors. MEK inhibitor–associated retinopathy may present as blurred vision, photophobia, transient visual disturbances, and subretinal fluid 47, 48. Get the full story with Premium Access Only $95 for the first 90 days* Premium Access gives you:.
MEK inhibitors (e.g., refametinib, selumetinib, trametinib, cobimetinib) have been tested in clinical trials for the treatment of NSCLC.92 The most common adverse effects of MEK inhibitors (e.g., trametinib) are rash, diarrhea, peripheral edema, fatigue, and dermatitis acneiform. It is usually associated with minimal symptoms and often does not require adjustments in therapy. Skin cancers are actually less common with the combination of BRAF and MEK inhibitors.
BRAF/MEK inhibitors have been highly successful among patients who harbor BRAF V600E mutations — present in between 30% to 50% of patients with melanoma. Trametinib Trametinib is TGA and PBS approved in Australia to treat melanoma, but there have been some encouraging results in treating plexiform neurofibromas and optic nerve gliomas in patients with NF1. MEK inhibitors (e.g., refametinib, selumetinib, trametinib, cobimetinib) have been tested in clinical trials for the treatment of NSCLC.
Both BRAF and MEK inhibitors have therefore been combined with different agents, the most successful treatment so far, however, was the combination with each other. 6,37 Although the. Add to Cart Quick View Add to Compare.
Rash, nausea, diarrhea, swelling, and sensitivity to sunlight. This marks the third BRAF/MEK inhibitor combination therapy approved for advanced melanoma. Efficacy of BRAFi + MEKi associates with cancer cell death and alterations in the tumor immune microenvironment;.
Bay43-9006 disables the B-Raf kinase domain by locking the enzyme in its inactive form. Acne, rosacea, dry skin, itching, rash,. The MEK inhibitor binimetinib in combination with the BRAF inhibitor encorafenib is in clinical development.
MEK inhibitor at varying concentrations was added to the cells in triplicate on day 0. MEK inhibitors are a family of drugs that slow or block the activity of specific mitogen-activated protein kinsase (MEK) enzymes, which are involved in cell production. These are allosteric binding inhibitors of.
The MEK Inhibitors and mTOR inhibitors, like Everolimus, each owe their success slowing down the pathway at different points. PD) paradoxically induce MEK phosphorylation (pMEK) by relieving ERK-dependent feedback inhibition of RAF which may limit their efficacy. The use of MEK inhibitors is likely to increase substantially in NF1.
In contrast to other MEK inhibitors in development, VS-6766 is a dual RAF/MEK inhibitor that blocks both MEK kinase activity and the ability of RAF to phosphorylate MEK. In addition to toxic effects common to small-molecule kinase inhibitors—such as rash, fatigue, and diarrhoea—toxicity events unique to MEK inhibitors, specifically ocular toxic effects that manifest as blurred vision and loss of visual acuity, have been described. Despite demonstrating in vitro activity, major in vivo limitations were identified for the early first generation MEK inhibitors, PD 37 and U0126.
A Trail of CDK4 / 6 Inhibitor and MEK Inhibitor in the Treatment of Metastatic Digestive System Tumors. Food and Drug Administration (FDA) approved the use of the BRAF inhibitor encorafenib and the MEK inhibitor binimetinib for the treatment of patients with metastatic or otherwise unresectable melanoma harboring a BRAF V600E/K mutation. This inhibition leads to cell death and the inhibition of tumor growth.
Mek inhibitors can have these side effects:. This small molecule/inhibitor is primarily used for Phosphorylation & Dephosphorylation applications. MEK, also known as Mitogen-activated protein kinase kinase and MAP2K, is a kinase enzyme that phosphorylates mitogen activated protein kinases (), ERK, p38 and JNK.Seven MEK subtypes have so far been identified;.
BI- is an orally bioavailable, and selective dual MEK/Aurora kinase inhibitor with IC50 of 3 nM, 25 nM, 15 nM, 25 nM, and 4 nM for Xenopus laevis Aurora B, human Aurora A and Aurora C, as well as human MEK1 and MEK2, respectively. PD is MEK inhibitor and non-competitive with ATP, Kiapp of 1 nM against activated MEK1 and MEK2. In a phase I/II trial, 150 mg of the BRAF inhibitor dabrafenib plus 2 mg/d of the MEK inhibitor trametinib resulted in a 76% ORR and impressive durable responses ( 1 ).
93 MEK inhibitors also have unique cardiac and ophthalmologic side effects. Combinations of BRAF inhibitors and MEK inhibitors (BRAFi + MEKi) are FDA-approved to treat BRAF V600E/K -mutant melanoma. Mekinist (trametinib) is a kinase inhibitor used to treat patients with melanoma with BRAF V600E or V600K mutations that are metastatic or unable to be removed by surgery (unresectable).
Given these changes, the Clinical Care Advisory Board of the Children's Tumor Foundation has identified a need within the NF1 clinical community for guidance for the safe and effective use of MEK inhibitors for NF1‐related tumors. Furthermore, it appears to have markedly. 93 MEK inhibitors also have unique cardiac and ophthalmologic.
Other MEK inhibitors have demonstrated preliminary activity in plexiform neurofibromas, providing reason to believe that other drugs in the class might also work, Widemann added. Since 11, the specific BRAF targeted agents, vemurafenib and dabrafenib, and the MEK inhibitor, trametinib, have been licensed for the treatment of patients with unresectable or metastatic BRAF mutant melanoma. The MEK Inhibitor II, also referenced under CAS -52-0, controls the biological activity of MEK.
Published October 26, by MEK Inhibitor- sgkinhibitor N (FK 506 (1M) and Cyclosporine A (CsA, 5M), Sigma) and sarco/endoplasmic reticulum Ca2ATPase (SERCA) (Thapsigargin, TSG, 10nM) have been also made use of. Approximately half of all melanomas carry a specific BRAF mutation known as V600E. The MEK gene works together with the BRAF gene, so drugs that block MEK proteins can also help treat melanomas with BRAF gene changes.
MEK1 and MEK2 activate ERK, MEK3 and MEK4 activate p38 and MEK5 and MEK6 activate JNK. BAY43-9006 (Sorafenib, Nexavar) is a V600E mutant B-Raf and C-Raf inhibitor approved by the FDA for the treatment of primary liver and kidney cancer. As with other biological targeted agents, these drugs are associated with predictable patterns of adverse events.
Mirdametinib (PD) is a selective and non ATP-competitive MEK inhibitor with IC50 of 0.33 nM in cell-free assays, roughly 500-fold more potent than CI-1040 on phosphorylation of ERK1 and ERK2. In general, MEK inhibitors are well tolerated. Oct 12, (The Expresswire) -- Global Targeted Drug MEK Inhibitors for NSCLC Market is a comprehensive research that provides information regarding.
The designation is for the treatment of pediatric patients aged three years and older with neurofibromatosis type 1 (NF1) symptomatic and/or progressive, inoperable plexiform neurofibromas (PN), a rare, incurable genetic condition. Trametinib is the first MEK inhibitor to be approved by the FDA for the treatment of melanoma, alone and in combination with the BRAF inhibitor, dabrafenib. MEK inhibitor | C26H26N4O2 | CID - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities.
The addition of an anti-PD-1/PD-L1 agent, such as pembrolizumab, durvalumab or atezolizumab, to combined BRAF and MEK inhibition has shown considerable promise, with several trials ongoing in metastatic melanoma. “Combined BRAF/MEK inhibitor therapy is standard of care in advanced BRAF V600–mutant melanoma, but approved combinations have unique toxicities that may impact the ability to deliver optimal treatment (ie, vemurafenib/cobimetinib Cotellic is associated with photosensitivity).” Go to full article published by The ASCO Post on Sep 10, 18. What Are Side Effects of Mekinist?.
The two most common MEK Inhibitor drugs that have been used to treat NF1 patients are:. A MEK inhibitor is a chemical or drug that inhibits the mitogen-activated protein kinase kinase enzymes MEK1 and/or MEK2. Trametinib (MEK inhibition) is the first medical therapy to have had any observable effect in primary NRAS-driven CNS melanoma in children in our cohort.
However, the links are poorly understood. Mirdametinib is designed to inhibit MEK1 and MEK2. It has been shown that the effective inhibition of the MAPK pathway through a combination of BRAF and MEK inhibitor therapy resulted in better clinical outcomes, 17 and it is known to ameliorate BRAF inhibition–related adverse events caused by MEK hyperproduction, like squamous skin cancer and other skin-related toxicities.
These data give Verastem more confidence in its plan to advance VS-6766, a MEK and pan-RAF inhibitor, into two registration-enabling studies in lung and ovarian cancer by the end of the year. The most common approach is to combine a MEK inhibitor with a BRAF inhibitor in BRAF mutation disease. CI-1040 (PD) was the first MEK inhibitor to.
The inhibition of the RAS/RAF/MEK/ERK pathway has long been an attractive therapeutic target for cancer treatment, particularly for the treatment of KRAS-mutant, NRAS-mutant, and BRAF-mutant cancers:. Common side effects can include:. MEK162 (ARRY-, Binimetinib) MEK162 (ARRY-) is a potent, selective, ATP uncompetitive inhibitor of MEK1/2.
In February 18, the FDA granted selumetinib an orphan drug designation for the treatment of NF1, and the European Medicines Agency also granted an orphan designation to the agent in August 18. MEK inhibitors are pills taken once or twice a day. These drugs can be used to treat melanoma that has spread or can’t be removed completely.
The cells were incubated at 37°C for 3 hours with MTT. Lupin’s proprietary MEK inhibitor compound is planned for clinical development in combination with Boehringer Ingelheim’s emerging KRAS inhibitor pipeline to address KRAS-driven cancers Lupin to receive a $ million upfront payment with potential total milestones of more than $700 million and royalties on the sales of the product. The RAS pathway can also be inhibited by natural products;.
The combination of the RAF-MEK inhibitor VS-6766 (CH) and the FAK inhibitor defactinib (VS-6063) elicited early signals of clinical activity in a group of patients with KRAS-mutant advanced. The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. They can be used to affect the MAPK/ERK pathway which is often overactive in some cancers.
RAS proteins are small GTPases that act as molecular switches controlling signaling pathways regulating cell proliferation and cell survival. Selumetinib inhibits MEK to prevent tumor growth due to dysregulations in RAS/RAF/MEK/ERK pathway signaling as a result ofNF1mutations. MEK inhibitors bind to and inhibit MEK, inhibiting MEK-dependent cell signaling.
Listing a study does not mean it has been evaluated by the U.S. Mirdametinib is an oral, small molecule MEK inhibitor in development as a monotherapy treatment for neurofibromatosis type 1‑associated plexiform neurofibromas, or NF1‑PN, and as a combination therapy for the treatment biomarker defined metastatic cancers with mutations in the MAPK pathway, such as in RAS and RAF. Common side effects of Mekinist include:.
MEK inhibitors (inhibiting targets of signaling pathways) used for various assays, some have entered clinical trials, which would be new cancer therapies. Upon activation, MAPKs can phosphorylate a variety of intracellular targets including. MTT dissolved in 0.8% NaCl solution at 5 mg/mL was added to each well (0.2 mL) on day 2 to test GI 50 or every day for cell growth curves.
Food and Drug Administration last week granted breakthrough therapy designation for the MEK 1/2 inhibitor selumetinib. 38 Although both compounds were deemed unsuitable for clinical consideration they have proven to be invaluable tools for investigating the Ras/MAPK pathway.

Proposed Model Involving Dusp6 For Response To Mek Inhibitor Download Scientific Diagram

Overcoming Resistance To Braf And Mek Inhibitors By Simultaneous Suppression Of Cdk4 Intechopen
Ocular Side Effects Of Mek Inhibitors Fluid Foci American Academy Of Ophthalmology
Mek Inhibitor のギャラリー

Nanosized Modification Strategies For Improving The Antitumor Efficacy Of Mek Inhibitors Bentham Science

Targeting Braf Mek Treatment Of Melanoma

Clinical Development Of Braf Plus Mek Inhibitor Combinations Trends In Cancer

Raf Suppression Synergizes With Mek Inhibition In Kras Mutant Cancer Cells Sciencedirect

Full Text Mek Inhibitors And Their Potential In The Treatment Of Advanced Melano Dddt

Ets Targeted Therapy Can It Substitute For Mek Inhibitors Clinical And Translational Medicine Full Text

Azd6244 Selumetinib Mek Inhibitor Xcessbio Biosciences Inc

Mechanisms Of Intrinsic Resistance To Braf Mek Inhibitors Coexistent Download Scientific Diagram
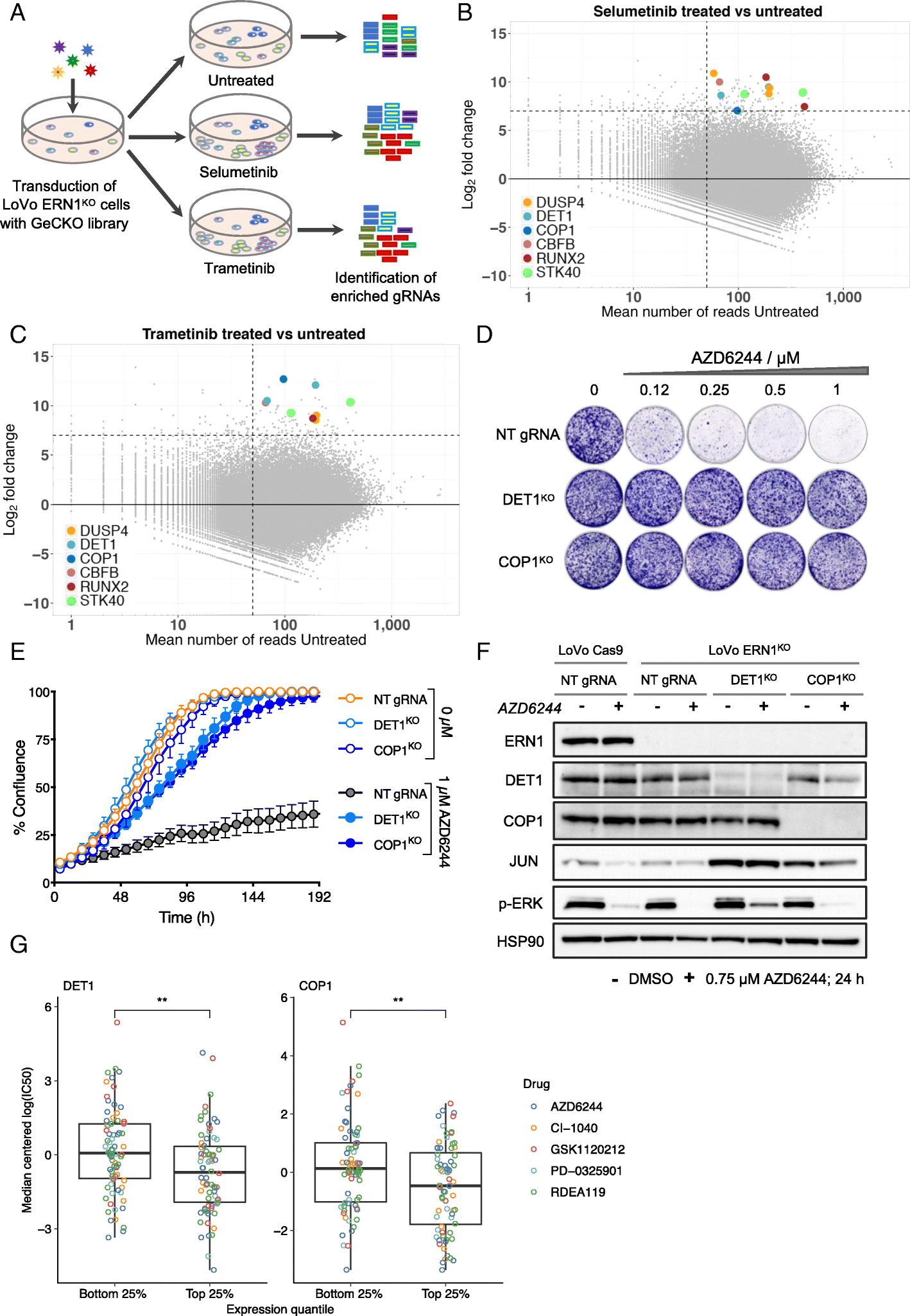
A Role For The Unfolded Protein Response Stress Sensor Ern1 In Regulating The Response To Mek Inhibitors In Kras Mutant Colon Cancers Genome Medicine Full Text

Histone Deacetylase Inhibitors A Promising Partner For Mek Inhibitors In Uveal Melanoma Melanoma Management

Oncology Nursing Society Cjon

High Throughput Screening Reveals Higher Synergistic Effect Of Mek Inhibitor Combinations In Colon Cancer Spheroids News Break
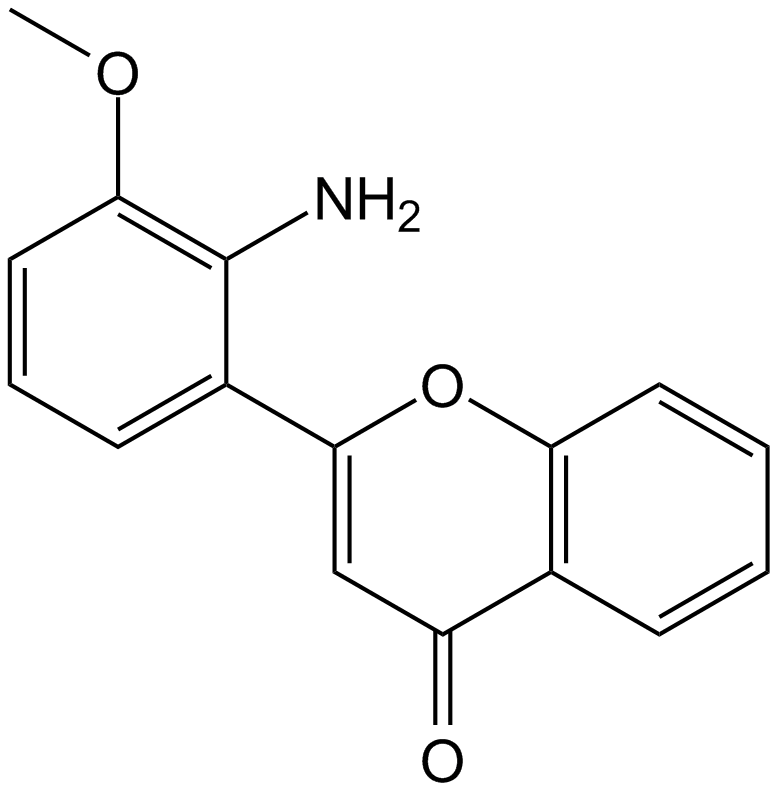
Apexbio Pd Mek Inhibitor Selective And Reversible Cas 21 8
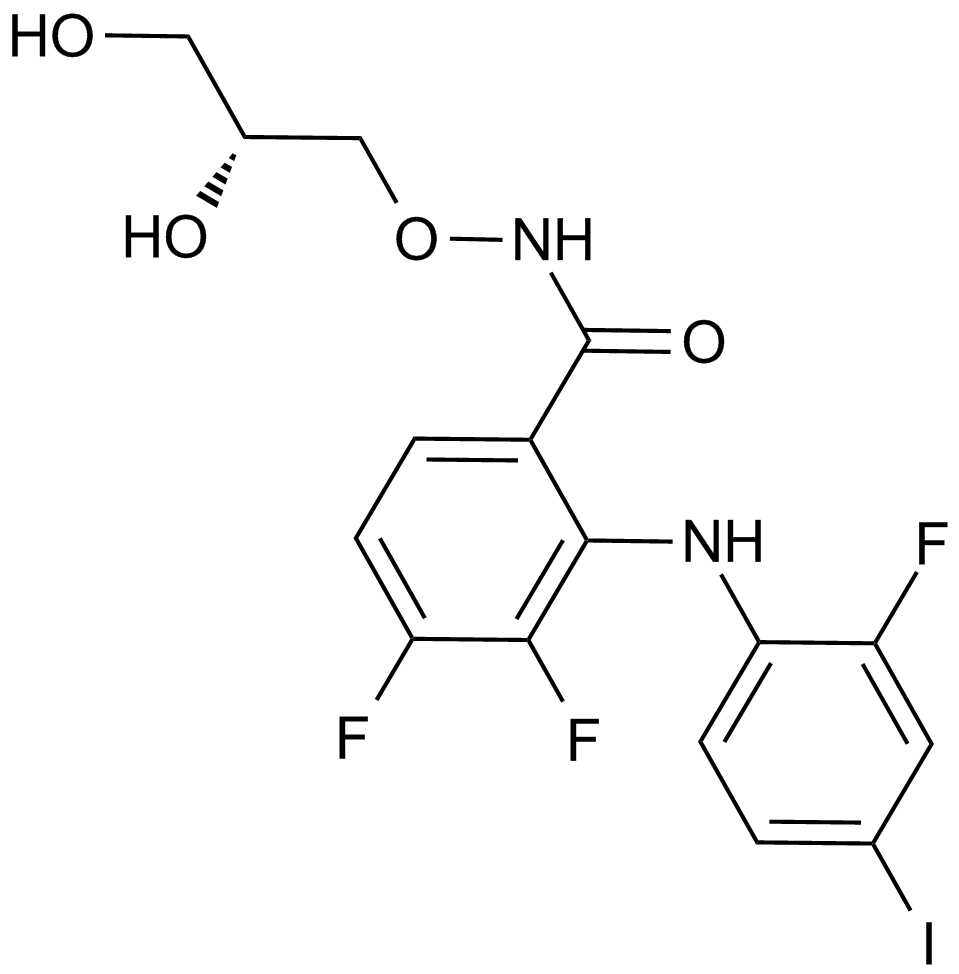
Apexbio Pd Mek Inhibitor Cas 10 9

Tf8mjkoebadgm
Q Tbn 3aand9gcrr6vtpqkipsi Hhvmo6crs C03s Ubm3gtvrwdu51cfpuwdugm Usqp Cau

Oncology Nursing Society Cjon
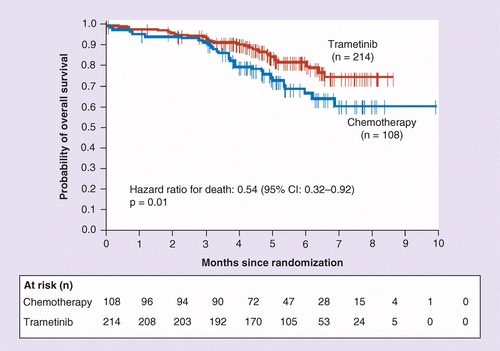
Role Of The Mek Inhibitor Trametinib In The Treatment Of Metastatic Melanoma Future Oncology
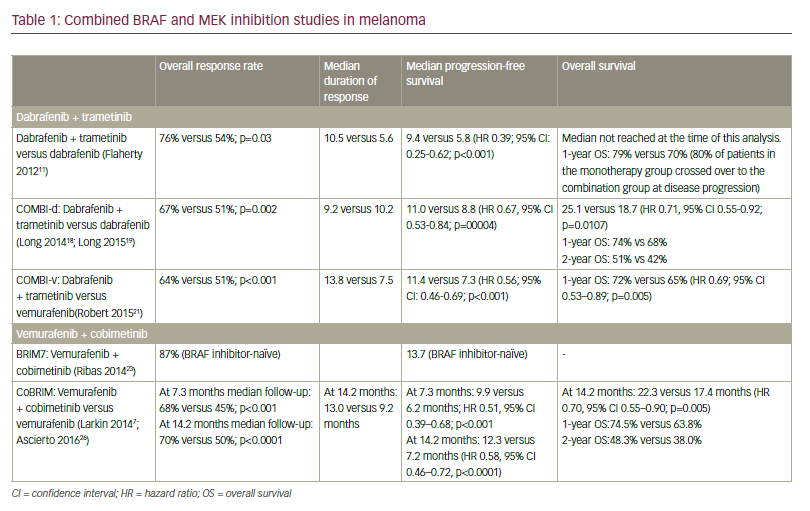
Combined Braf And Mek Inhibition For Patients With Advanced Melanoma

Mek Inhibitor I Cas 42 4 Scbt Santa Cruz Biotechnology

Safety Lead In Of The Mek Inhibitor Trametinib In Combination With Gsk An Akt Inhibitor In Patients With Recurrent Endometrial Cancer An Nrg Oncology Gog Study Gynecologic Oncology

Intracranial Antitumor Activity With Encorafenib Plus Binimetinib In Patients With Melanoma Brain Metastases A Case Series Holbrook Cancer Wiley Online Library

Figure 2 From Cardiovascular Effects Of The Mek Inhibitor Trametinib A Case Report Literature Review And Consideration Of Mechanism Semantic Scholar
Www Tandfonline Com Doi Pdf 10 1080 17
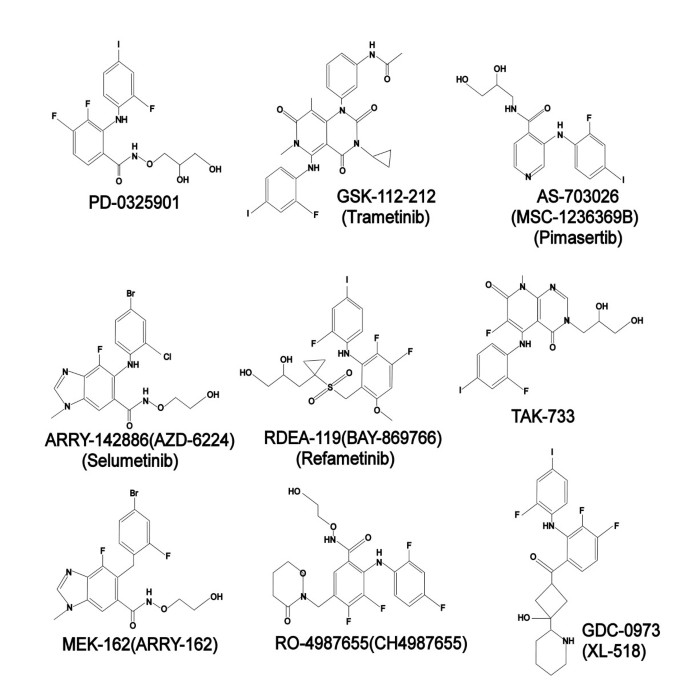
Mek And The Inhibitors From Bench To Bedside Journal Of Hematology Oncology Full Text

Mek Inhibitor Set Calbiochem The Mek Inhibitor Set Controls The Biological Activity Of Mek This Small Molecule Inhibitor Is Primarily Used For Phosphorylation Dephosphorylation Applications Sigma Aldrich

Pd Mek Inhibitor
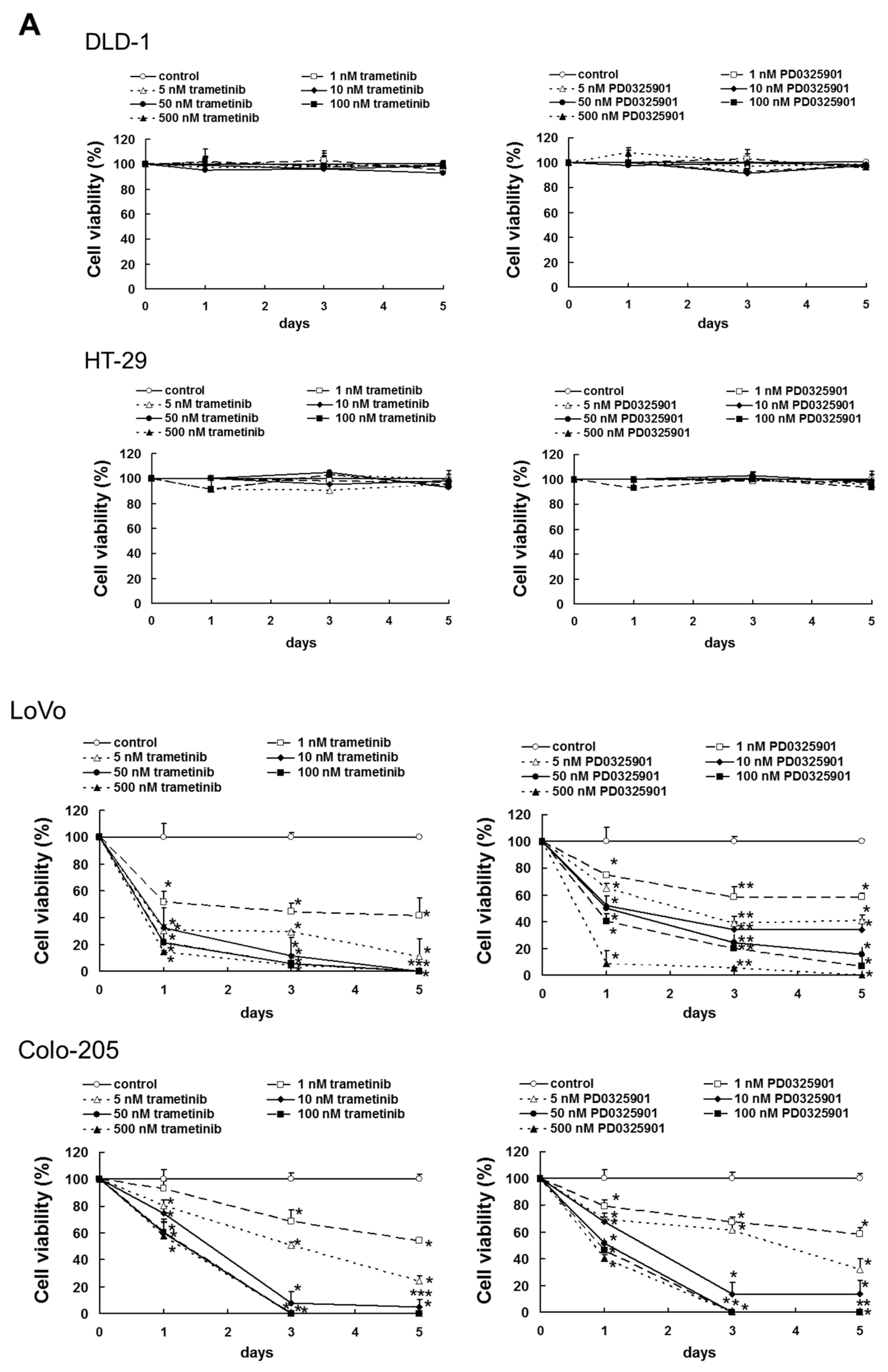
Cancers Free Full Text Overactivation Of Akt Contributes To Mek Inhibitor Primary And Acquired Resistance In Colorectal Cancer Cells
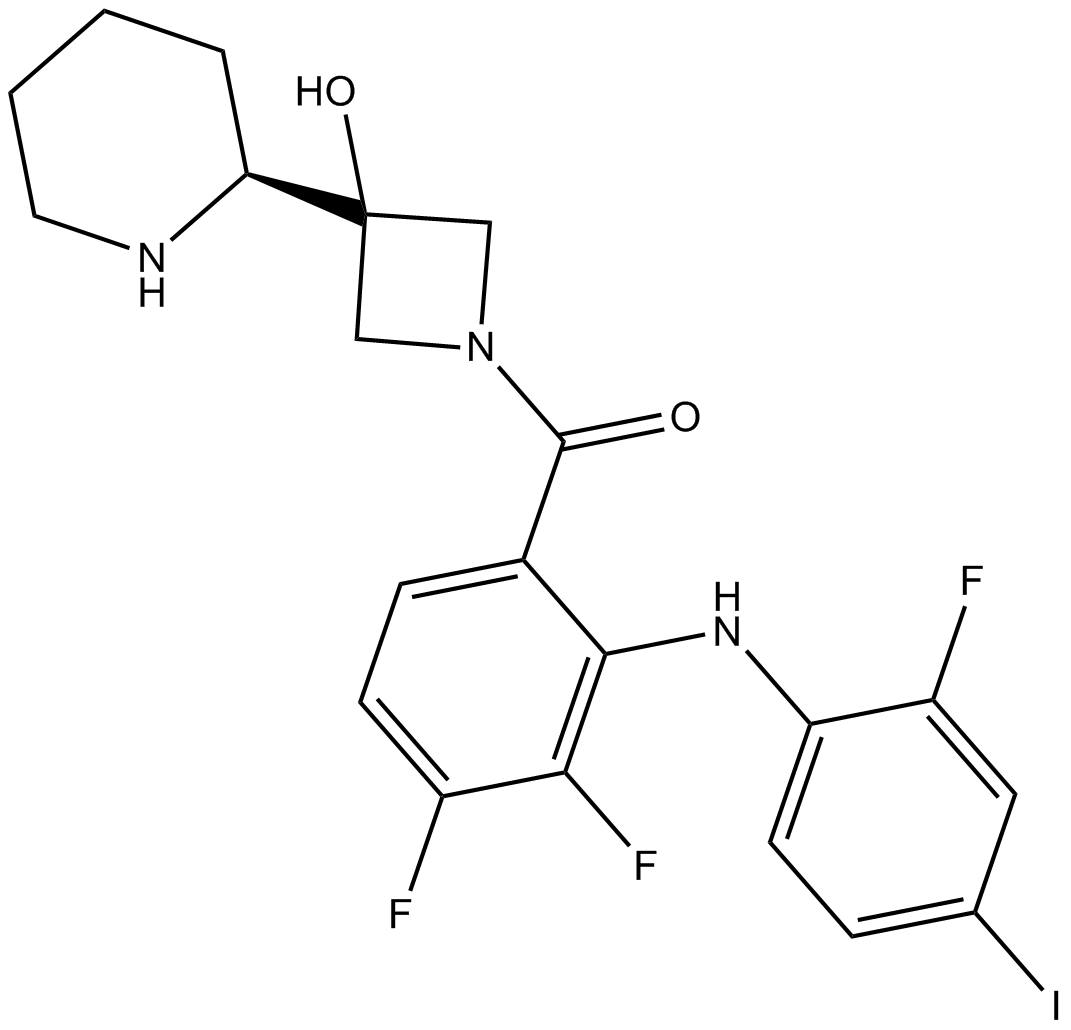
Apexbio Cobimetinib Selective Mek Inhibitor Cas 93 2

Mek Inhibitors As A Novel Therapy For Neuroblastoma Their In Vitro Effects And Predicting Their Efficacy Sciencedirect

Jci Anticancer Therapy Boosting The Bang Of Bim

Mek Inhibition Induces Myog And Remodels Super Enhancers In Ras Driven Rhabdomyosarcoma Science Translational Medicine
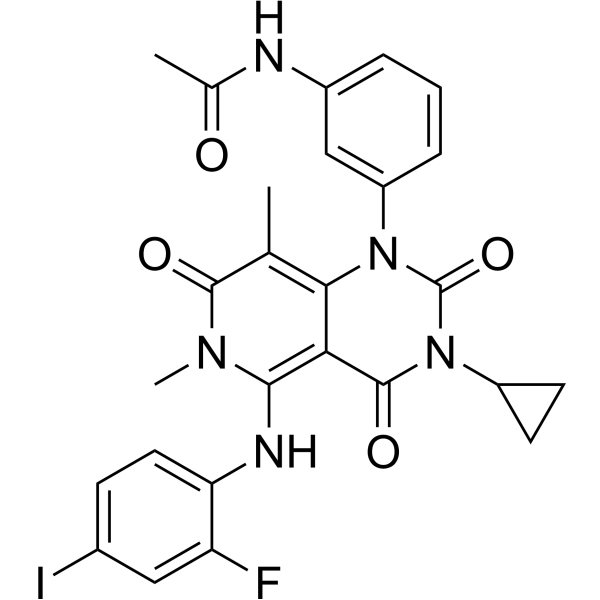
Trametinib Gsk Mek Inhibitor Medchemexpress
Case Study Mek Inhibitor Ntrc Oncolines
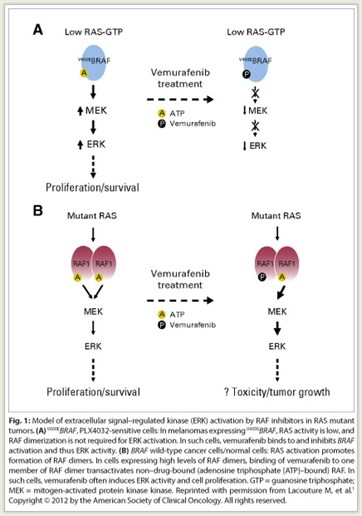
Answer To Secondary Cancers With Raf Inhibitors May Be Concomitant Mek Inhibition The Asco Post
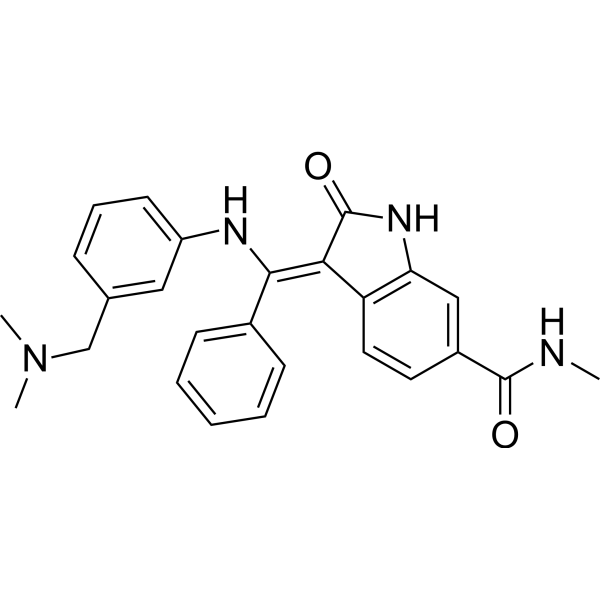
Mek Inhibitor Mek Inhibitor Medchemexpress

The Clinical Development Of Mek Inhibitors Semantic Scholar

Targeting Alterations In The Raf Mek Pathway Cancer Discovery
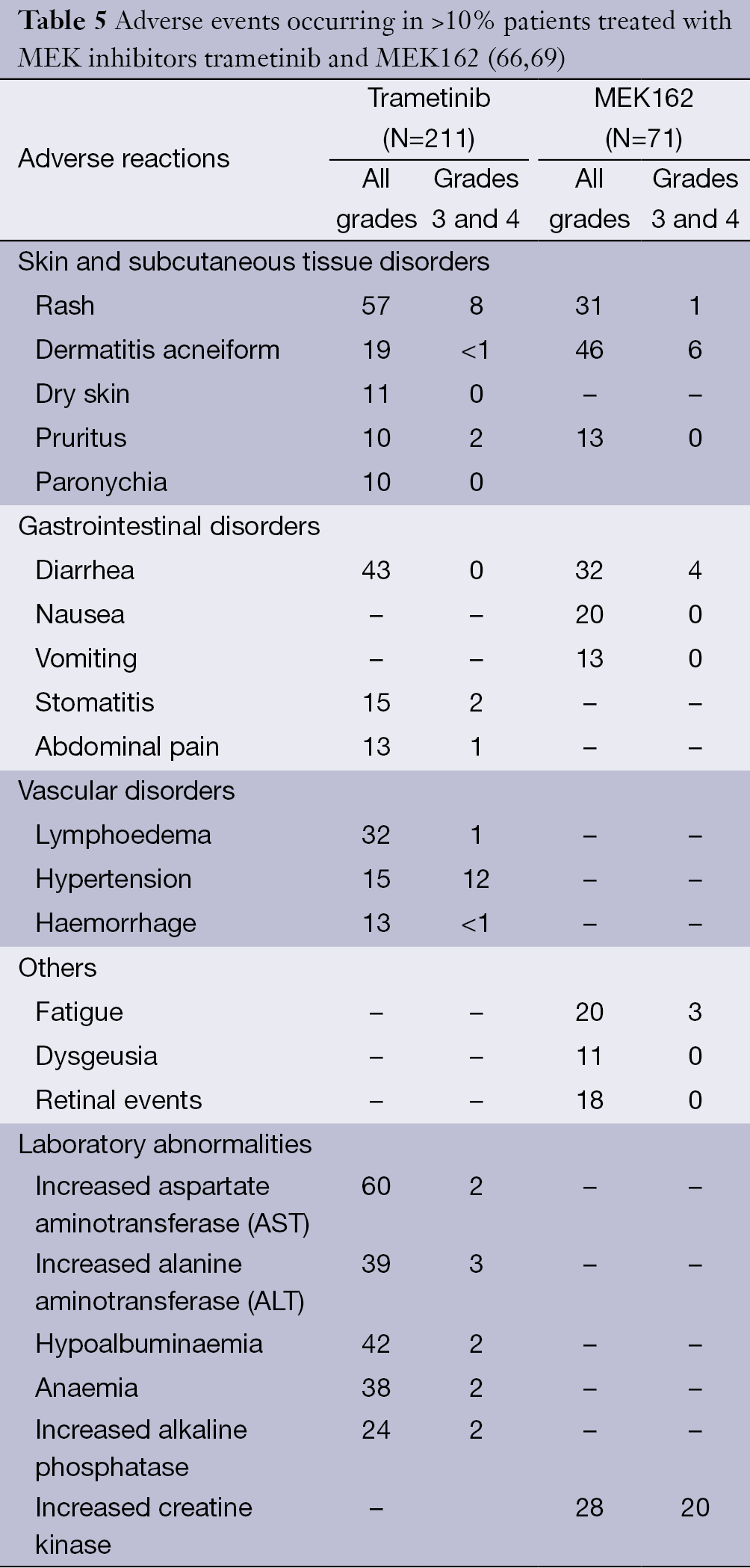
Braf Mek And Kit Inhibitors For Melanoma Adverse Events And Their Management Livingstone Chinese Clinical Oncology
Www Jpedsurg Org Article S0022 3468 16 9 Pdf

Mek Inhibitor Investigational Compounds Inoncology
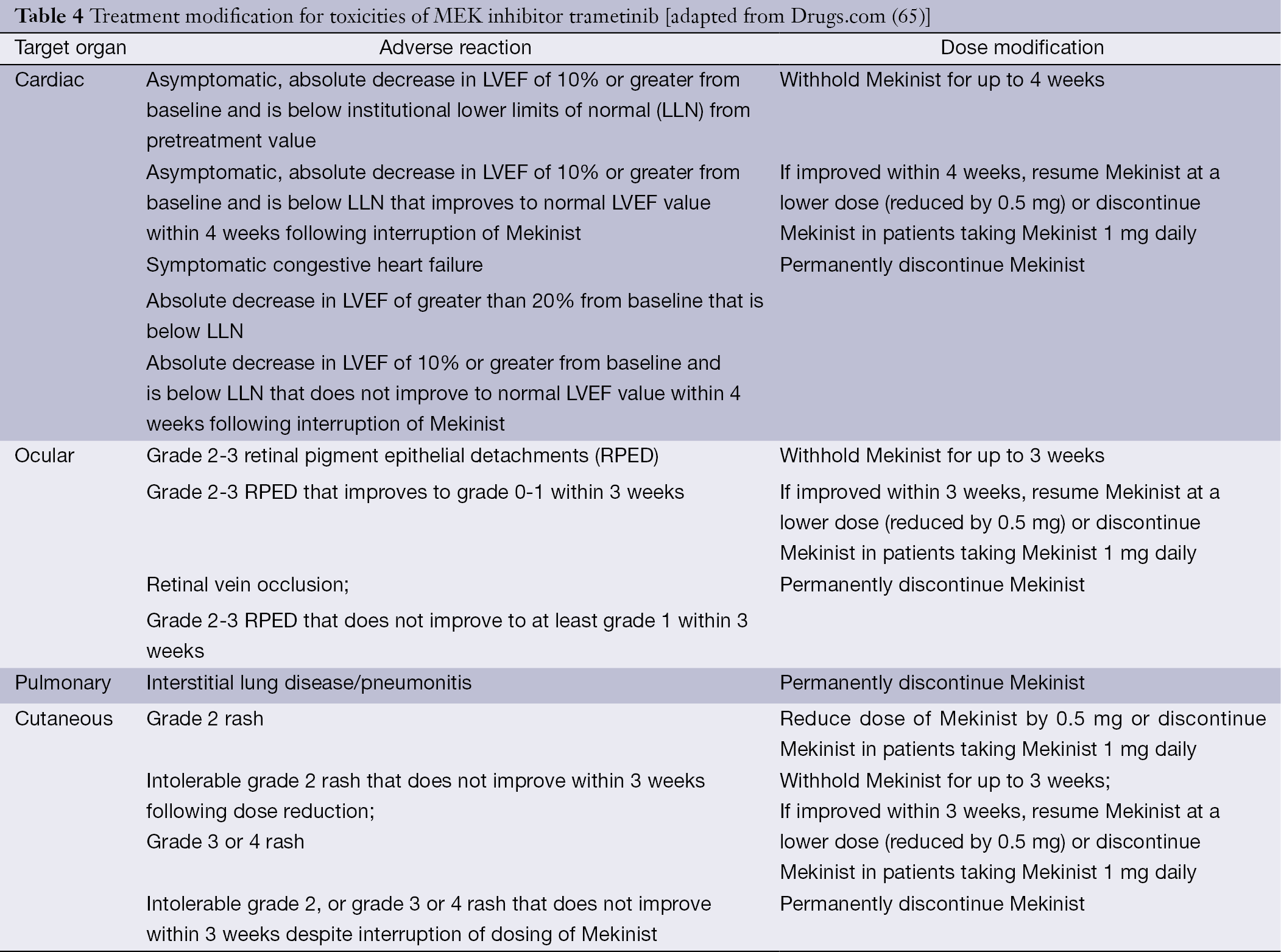
Braf Mek And Kit Inhibitors For Melanoma Adverse Events And Their Management Livingstone Chinese Clinical Oncology

Tolerability Of Braf Mek Inhibitor Combinations Adverse Event Evaluation And Management Esmo Open
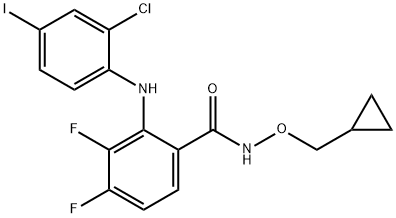
Mek Inhibitor
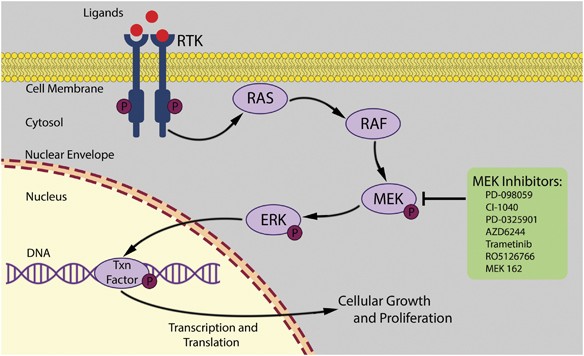
Mek Inhibitors A New Class Of Chemotherapeutic Agents With Ocular Toxicity Eye
Plos One Molecular Subtype Specific Efficacy Of Mek Inhibitors In Pancreatic Cancers

Novel Atp Competitive Mek Inhibitor E61 Is Effective Against Vemurafenib Resistant Melanoma Harboring The Mek1 C121s Mutation In A Preclinical Model Molecular Cancer Therapeutics
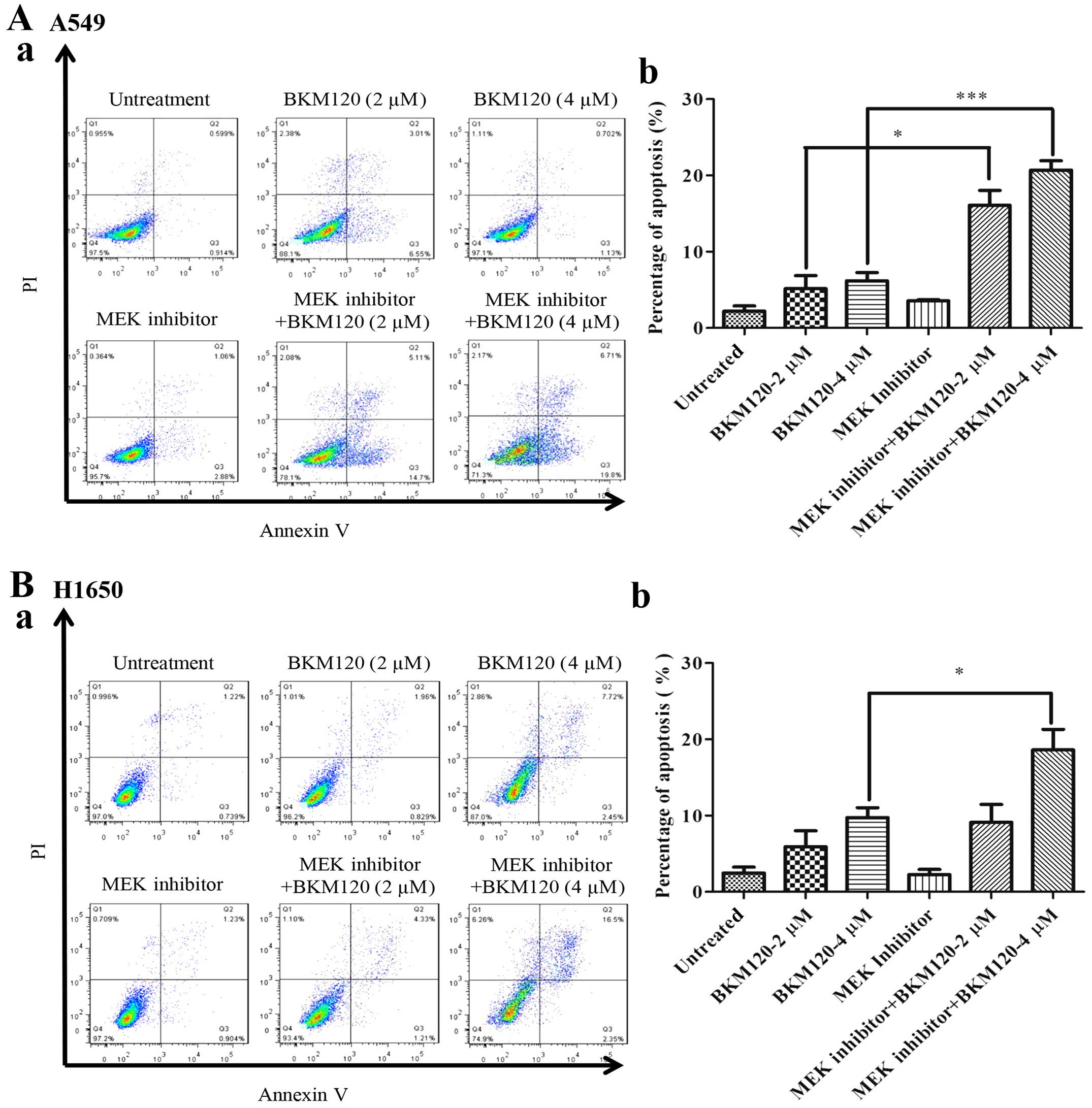
Combined Use Of Pi3k And Mek Inhibitors Synergistically Inhibits Lung Cancer With Egfr And Kras Mutations

Mek Inhibitor Mek Inhibitor Buy Mek Inhibitor From Supplier Adooq

The Mapk Pathway And The Role Of Braf And Mek Inhibitors Download Scientific Diagram
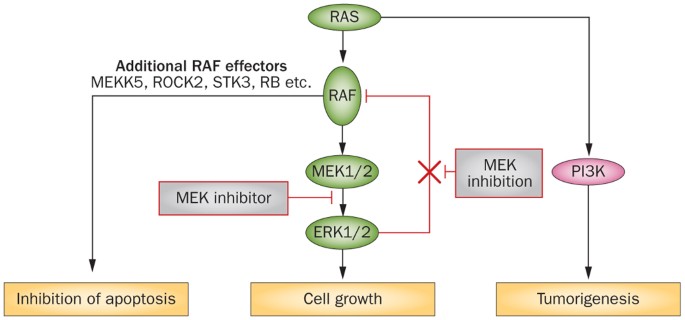
The Clinical Development Of Mek Inhibitors Nature Reviews Clinical Oncology
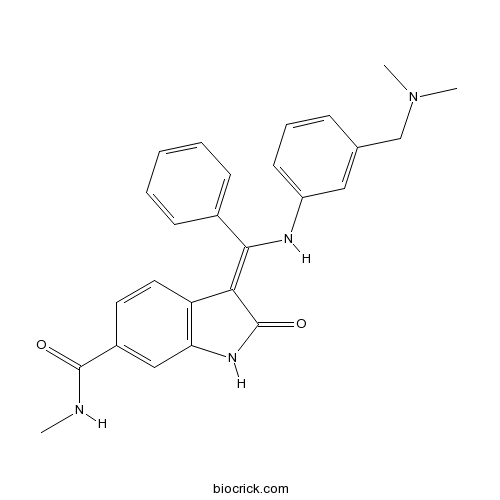
Mek Inhibitor Cas 92 7 Potent Mek Inhibitor Antitumor Agent High Purity Manufacturer Biocrick

Mek Inhibitor Ii Cas 52 0 Calbiochem The Mek Inhibitor Ii Also Referenced Under Cas
Trametinib Is A Cancer Drug It Is A Mek Inhibitor Drug With Anti Cancer Activity 3d Illustration Stock Photo Alamy

Trametinib A Mek Inhibitor For Management Of Metastatic Melanoma Oncology Nurse Advisor

Molecular Mechanisms Of Resistance To Braf And Mek Inhibitors In Brafv600e Non Small Cell Lung Cancer European Journal Of Cancer

Mek Inhibition Enhances Oncolytic Virus Immunotherapy Through Increased Tumor Cell Killing And T Cell Activation Science Translational Medicine
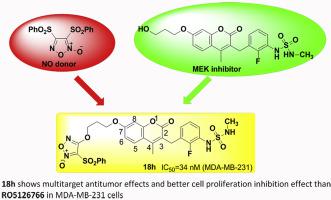
Hybrids Of Mek Inhibitor And No Donor As Multitarget Antitumor Drugs Eur J Med Chem X Mol

Resistance To Combination Braf And Mek Inhibition In Metastatic Melanoma Where To Next Sciencedirect

Mek Inhibitor Mechanism Of Action Side Effects And Uses

Intrinsic Resistance To Mek Inhibition In Kras Mutant Lung And Colon Cancer Through Transcriptional Induction Of Erbb3 Sciencedirect

Mek Inhibition Induces Distinct Feedback Activation Of Rtks Based On Download Scientific Diagram

Milliporesigma Calbiochem Mek Inhibitor Set 1 Set Life Sciences Fisher Scientific
Plos One Mek Inhibition Suppresses B Regulatory Cells And Augments Anti Tumor Immunity
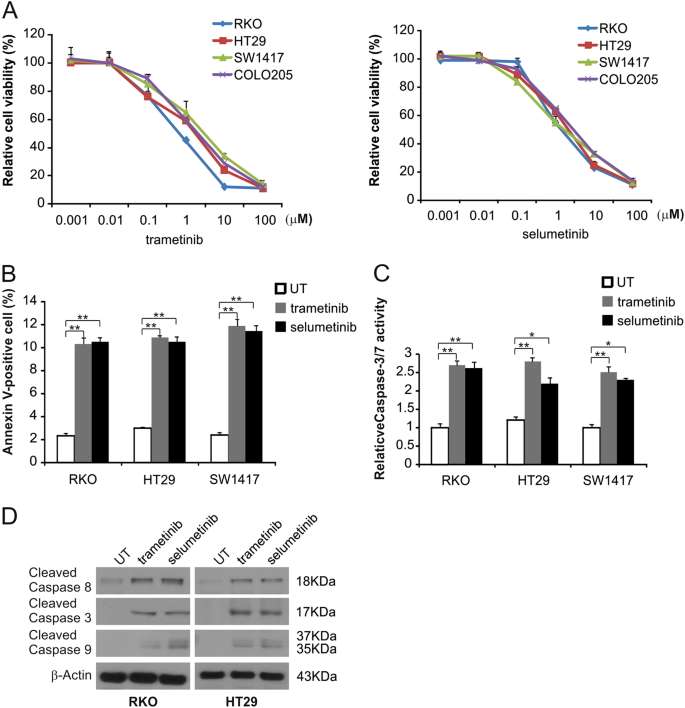
Mek Inhibitors Induce Apoptosis Via Foxo3a Dependent Puma Induction In Colorectal Cancer Cells Oncogenesis

Pd Mek Inhibitor Cellagen Technology

Division Of Molecular Therapeutics Research Divisions Aichi Cancer Center Research Institute
How Cancer Cells Dodge Targeted Silver Bullet Treatments Braf Mek Resistance In Melanoma Oncobites
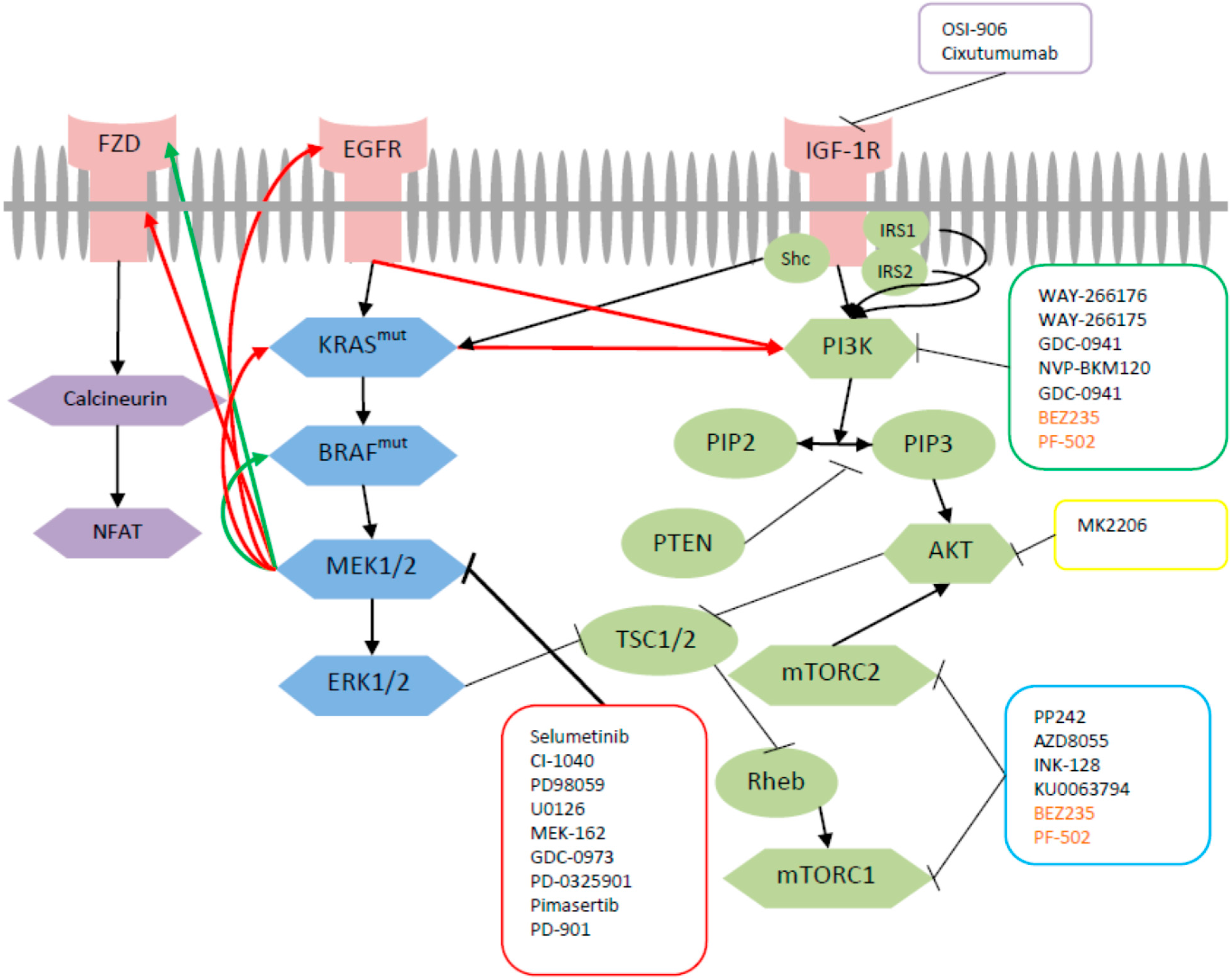
Ijms Free Full Text Dual Inhibition Of Mek And Pi3k Pathway In Kras And Braf Mutated Colorectal Cancers

New Possibility Of Molecularly Targeted Therapy For Lung Cancer With Kras Gene Mutation Kanazawa University

Mechanisms Of Acquired Resistance To Braf Mek Inhibitors Multiple Download Scientific Diagram

Milliporesigma Calbiochem Mek Inhibitor Ii 5mg Life Sciences Fisher Scientific

Mek Inhibitor Investigational Compounds Inoncology

Effect Of Mek Inhibitor I On Co Culture Of Gastric Cancer Cells With Gastric Stromal Cells
Q Tbn 3aand9gctzbqajlvavbivxqa3p06mieuy2ti2a63ha0ym6hmewnl1mo8kv Usqp Cau

Trametinib Is A Cancer Drug It Is A Mek Inhibitor Drug With Stock Photo Picture And Royalty Free Image Image

A Case Of Non Resolving Mek Inhibitor Associated Retinopathy Happidoc
Synergistic Inhibition Of Mek And Reciprocal Feedback Networks For Targeted Intervention In Malignancy Li Cancer Biology Medicine

Selumetinib Wikipedia
Synergistic Inhibition Of Mek And Reciprocal Feedback Networks For Targeted Intervention In Malignancy Li Cancer Biology Medicine
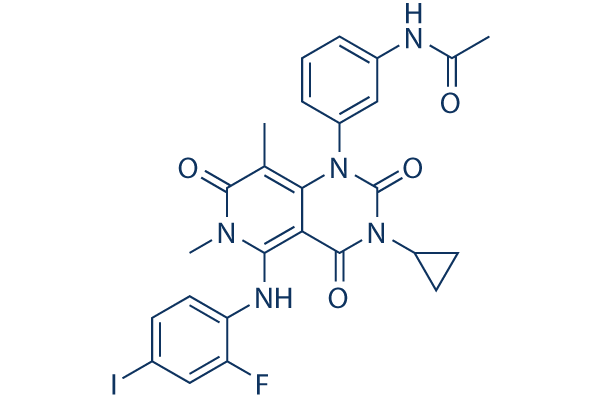
Trametinib Gsk 99 Hplc Selleck Mek Inhibitor

The Dynamics Of Erk Signaling In Melanoma And The Response To Braf Or Mek Inhibition Are Cell Cycle Dependent Biorxiv

Scheme Of The Mek Inhibitor Induced Crosstalk Mechanism A In Absence Download Scientific Diagram

Trametinib Is A Cancer Drug It Is A Mek Inhibitor Drug With Stock Photo Picture And Royalty Free Image Image

Molecular Pathways The Basis For Rational Combination Using Mek Inhibitors In Kras Mutant Cancers Clinical Cancer Research
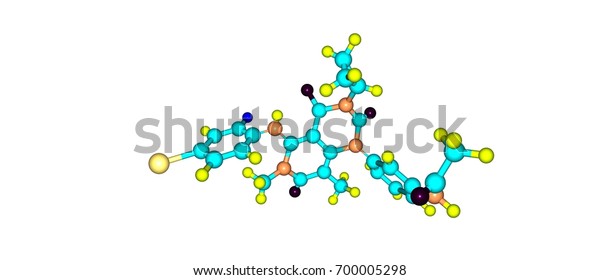
Trametinib Cancer Drug Mek Inhibitor Drug Stock Illustration

Oncogenic Pi3k Akt Promotes The Step Wise Evolution Of Combination Braf Mek Inhibitor Resistance In Melanoma Oncogenesis X Mol
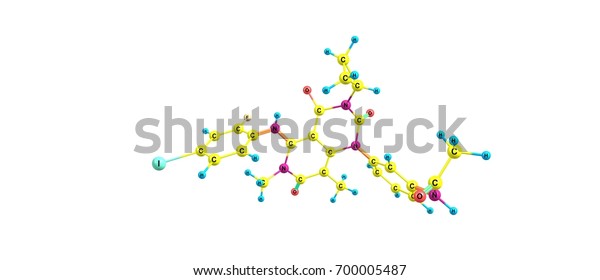
Trametinib Cancer Drug Mek Inhibitor Drug Stock Illustration
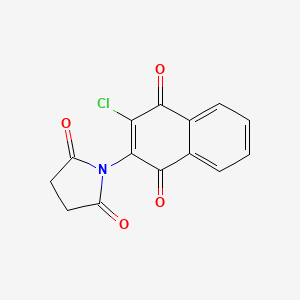
Mek Inhibitor Ii C14h8clno4 Pubchem
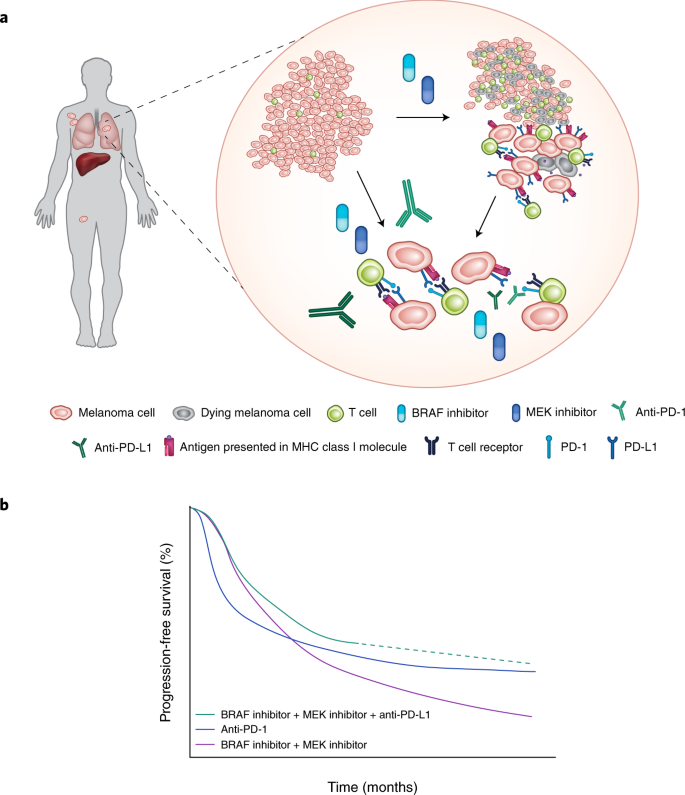
Combining Checkpoint Inhibition And Targeted Therapy In Melanoma Nature Medicine

Trametinib A Mek Inhibitor For Management Of Metastatic Melanoma Oncology Nurse Advisor

Full Text Mek Inhibitors And Their Potential In The Treatment Of Advanced Melano Dddt

Pharmacodynamic Elegance Combination Of Braf And Mek Inhibitors Capho
Mek Inhibitor I C21h18n4os Chemspider
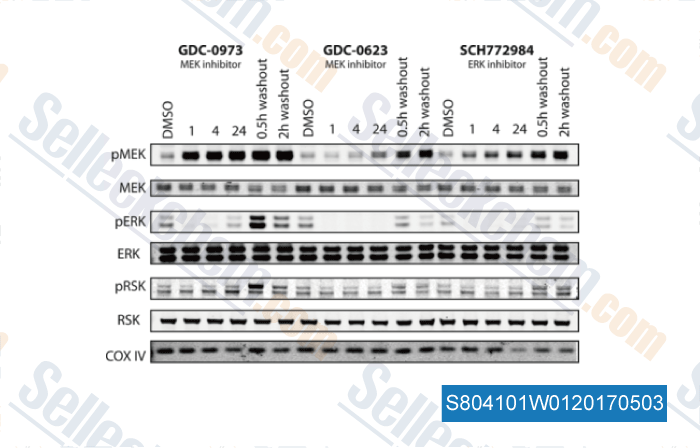
Mek Inhibition Mek Inhibitor Review

Top Pdf Mek Inhibitor 1library
Trametinib Is A Cancer Drug It Is A Mek Inhibitor Drug With Anti Cancer Activity 3d Illustration Stock Photo Alamy
U0126 Etoh 99 Hplc Selleck Mek Inhibitor

Overcoming Resistance To Dual Innate Immune And Mek Inhibition Downstream Of Kras Sciencedirect



